Mental health problems in the workforce represent a significant burden for the affected worker and its family, and for both businesses and the whole of society, given the costs associated with mental illness (absenteeism, lower productivity, indemnity payments, and health care) (1). Evaluation of workers” mental health is, however, a difficult task to achieve and may be riddled with controversy given competing interests between employers and employees. In attempting to develop screening tools that can detect impending invalidity (2, 3), psychiatric symptoms (such as psychological distress, depression, and burnout) are traditionally measured using self-report psychological and sociological questionnaires, while methodologies from the biological sciences have generally been applied more often to severe forms of mental disorders (4). Bridging information derived using bio-, psycho-, and sociometrics in combination is important given the frequent contention between workers and employers regarding the sources and severities of workplace stressors, psychological consequences, and compensation thereof (5). A promising trans-disciplinary approach that could help reconcile these issues and ultimately refine methods is to assess how psychological symptom severities are associated with biological signatures. The present study examines how self-reported psychological distress and depressive and burnout symptoms correspond with variations in cortisol concentrations at different time points over non-work and working days.
A rich research literature has focused on the biological functioning of distressed workers. Upon perceiving real or interpreted threats, the hypothalamic-pituitary-adrenal (HPA)-axis produces cortisol that synergistically mobilizes energy (6, 7). Individual differences in stress hormone profiles can be investigated via pharmacological and psychosocial paradigms in addition to more naturalistic approaches that assess circadian or diurnal variations. A normal diurnal cortisol rhythm consists of an acute increase during the first hour after awakening (8, 9) referred to as the cortisol awakening response (CAR). This peak is followed by gradual decreases throughout the day, reaching the lowest levels before bedtime. Beyond diurnal variation, cortisol concentrations tend to increase from non-work- to workdays (10–12). Time of the day and work/non-workdays are therefore important when evaluating cortisol profiles.
The overall directionality of cortisol concentrations in relation to workplace stress and psychiatric symptomatologies is complex and inconsistently reported, although an increasing number of reports have linked distinct diurnal cortisol time points to specific features. In a recent meta-analysis of 62 articles, the magnitude of the CAR was positively associated with job and general life stress, but negatively associated with fatigue, burnout, and exhaustion (13). This therefore suggests that psychological distress and depressive symptomatologies may be more associated with “hyper”-cortisolemic profiles, while burnout symptomatologies may be related to “hypo”-cortisolimic profiles (13), at least with regards to morning samples such as at awakening or calculations representing the CAR. Much less is known about cortisol profiles during the afternoon and evening in relation to psychiatric symptoms.
Given that psychiatric symptoms overlap considerably, this opens important avenues for researchers to apply triangulated methodologies (14) that combine psycho- and biometric information to understand complex phenomena. Importantly, the severity of psychiatric symptoms for ill-defined syndromes like burnout is essential, since at times results appear to be positively associated with awakening and morning cortisol among patient populations (15), and yet blunted cortisol concentrations are equally reported in sub-clinical and clinical populations (13). This ultimately highlights the importance of symptom severities in relation to specific diurnal time points that may differ from morning, afternoon, and evening levels. No consensus exists and more research must address whether specific psychological symptoms correspond to distinct circadian recalibrations of the HPA-axis among functional yet distressed workers.
Overall, studies assessing diurnal cortisol in relation to psychological distress and depressive and burnout symptoms highlight various associations with dysregulated cortisol profiles. Yet, these studies generally have small sample sizes, varying cortisol sampling designs and protocols, and generally focus on specific employment types (eg, nurses, teachers, managers) within specific companies that ultimately limit generalizability both nationally and internationally. The current study aims to rectify this by assessing the associations between psychological distress and depressive and burnout symptoms in relation to diurnal cortisol profiles in a representative sample of healthy day-shift workers randomly selected from diverse Canadian workplaces and industries.
Based on our interpretation of the literature, our main hypothesis was that sub-clinical psychological distress and depressive and burnout symptoms would be associated with specific time points of diurnal cortisol concentrations when controlling for an array of confounders. Given the paucity of data and mixed findings, we did not postulate specific directionality for morning, afternoon, and evening cortisol profiles. In addition, previous studies have identified important inequalities in the distribution of different occupations and the differential exposure to unique stressors within diverse workplaces that may render Canadian women more vulnerable than men to stress-related health problems (16–19). Accordingly, we assessed differences according to workplaces in our main analyses as well as the moderating effect of sex in secondary analyses.
Methods
Data
The SALVEO study collected data throughout 2009–2012 using a total sample comprised of 34 Canadian workplaces randomly selected from a list of >500 companies insured by a large insurance company. For each workplace, a random sample of employees was first selected to answer a questionnaire that included 300 questions pertaining to the individual, work conditions, occupation, family, and community characteristics. The original sample was composed of 1301 workers with an average response rate of 66.7% (range 55.3–95.5%).
From among these respondents, our goal was to recruit a sample of 10–15 workers per workplace to participate in the second phase of the research project whereby saliva samples were collected for assessment of cortisol concentrations. This approach has been successfully used in the past (12). In so doing, a random subsample of 1043 workers was re-invited in the current biomarker substudy, of which 401 workers agreed to participate (11.8 workers per workplace on average), for a response rate of 38.4%. Response rate between women (40.8%) and men (36.1%) was not statistically significant (χ2=2.50, df=1, P=0.114). Women represented 56.1% of workers and the mean age of the entire sample was 41.3 years [standard deviation (SD) 10.81, range: 19–69].
Participants signed an informed consent form and were given detailed instructions. The Ethics Committees of the University of Montreal, McGill University, Laval University, Bishop’s University, and Concordia University approved the study protocol for the first and second phase of this research project.
Saliva sampling and cortisol determination
Consenting workers were instructed to provide five saliva samples per day at the following occasions: (i) at awakening, (ii) 30 minutes after awakening, (iii) 14:00 hours, (iv) 16:00 hours, and (v) bedtime. Previous studies have validated that these sampling times are reliable markers of diurnal cortisol secretory patterns (20, 21). Sampling was repeated for three days (one rest day and two working days, these days being Saturday, Tuesday, and Thursday for the majority of workers) over the course of a week in order to provide ecological validity representative of differences that could occur in work and non-work contexts (22).
Thirty minutes before providing saliva samples, participants were instructed to not eat a major meal, smoke cigarettes, drink caffeinated beverages (eg, tea, coffee, soft drinks) or fruit juices, consume dairy products (eg, yoghurt, milk, cheese), and additionally asked to rinse their mouths with water to eliminate any lodged food deposits. They were further instructed to not brush their teeth, floss, or engage in strenuous physical activity two hours prior to sampling. Compliance was assessed using a logbook in which participants documented their collection times for each sample.
Saliva was collected using Salivettes (Sarstedt, Ville St-Laurent, Quebec, Canada) using the pure-spit method, whereby a small quantity of saliva is guided by a straw into a tube. Participants stored saliva samples in their home freezer until a research assistant retrieved the participants” samples at their workplace one week later. Samples were frozen at -20o C in a portable freezer and then stored in an industrial -20o C freezer until final cortisol determinations.
Salivary cortisol concentrations were analyzed at the Centre for Studies on Human Stress with a high sensitivity enzyme immune assay kit (Salimetrics® State College, PA, USA, Catalogue No. 1-3102). Frozen samples were brought to room temperature to be centrifuged at 15000 xg (3000 rpm) for 15 minutes. The total binding and non-specific binding cortisol proportions typically ranged between 47–63% and 0.5–1.5%, respectively. The intra- and inter-assay coefficient of variation for these studies were 4.6% and 5%, respectively. The range of detection for this assay is between 0.012–3 dl and is assayed in duplicates that are then averaged.
Mental health questionnaires
Psychological distress, depression, and burnout symptoms were evaluated with well-validated instruments several days before providing saliva samples. Psychological distress, depression, and burnout scales were used as continuous variables in the statistical analysis.
Psychological distress was measured with the General Health Questionnaire short-form 12-items (23) (GHQ-12, α=0.85). Each item (eg, unable to concentrate on whatever you’re doing, feeling constantly under strain) was measured on a 4-point scale (1=better than usual, 4=much worse than usual). As recommended (23), items were binary recoded (1–2=0, 3–4=12) before being summed.
Depression was measured with the Beck Depression Inventory 21-items (24) (BDI-21, α=0.91). Each item (eg, feeling sad, suicidal thoughts) was measured on a 4-point scale with different ordinal classifications according to the symptom severity evaluated.
Burnout was measured with the Maslach Burnout Inventory 16-item General Survey (25) (MBIGS-16, α=0.89). Each item (eg, feeling emotionally drained from work, feeling used up at the end of the workday) was evaluated on a 7-point scale for symptom frequency (0=never; 6=daily). This study used a global burnout score that essentially combined the three subscales derived from this instrument; namely, emotional exhaustion, cynicism, and professional inefficacy (reverse coded from the original professional efficacy subscale).
Covariates
Based on previous reports (26) of their confounding effects on diurnal cortisol concentrations, analyses were adjusted for the following covariates: self-reported time of awakening, sex, age, season of sampling, cigarette smoking, alcohol consumption, regular physical activity, psychotropic drug use, physical health problems, and body mass index (BMI).
Sex was coded as binary (0=men, 1=women) and age was measured in continuous years. Season of sampling was coded into four categories (1=spring, 2=summer, 3=fall, 4=winter). Smoking was recorded continuously as the number of cigarettes smoked per day. For alcohol, respondents indicated the number of drinks they consumed on each of the seven days during the week preceding the administration of the questionnaire. Alcohol intake was measured by summing the number of drinks consumed daily (standard Canadian drink of 13.6 g/alcohol equivalents for beer, wine, and spirits) over the preceding week. Physical activity over the last three months was measured as the frequency of physical activities performed for >20 minutes. Respondents indicated this frequency on a 7-point Likert-type scale (1=never, 7=≥4/week). Prescribed psychotropic drugs over the last month were dichotomously coded (0=non-user and 1=user) for at least one the following: tranquilizers, antidepressants, codeine-demerol-morphine, and sleeping pills. Health problems status was tallied as the number of health problems lasting ≥6 months and diagnosed by a physician using a list of 29 items (eg, heart disease, cancer, asthma). BMI was computed as weight (kg) divided by height squared (m)2.
Statistical analysis
Comparison of respondents and non-respondents was first carried out to evaluate possible biases. Non-response or attrition analysis revealed non-significant differences between respondents (N=401) and non-respondents (N=642) regarding sex (P=0.21), age (P=0.08), psychological distress (P=0.06), depressive (P=0.09) and burnout (P=0.52) symptoms, season of sampling (summer P=0.90, fall (P=0.06, winter P=0.68), alcohol consumption (P=0.37), physical activity (P=0.27), psychotropic drugs use (P=0.15) and health problems (P=0.64). However, respondents smoked less (P<0.01) and had a lower BMI (P<0.01) than non-respondents.
In assessing missing data for questionnaires and a total of 6150 saliva samples, 5690 samples were analyzable in conjunction to complete information for psychological distress and depressive and burnout symptoms. The final sample of workers remaining was 396.
We next applied multilevel regression models (27, 28) to analyze cortisol concentrations in the following levels: (i) cortisol measurements at each occasion within a day at level 1, (ii) workers at level 2, and finally (iii) workplaces at level 3. In this manner, variations within a day were embedded within each unit of the second level, followed by variations between participants that were then embedded within each unit of the third level, and finally the variation between workplaces. This approach allows the full range of data to be taken into account when estimating cortisol variations across each level of the hierarchical data structure that furthermore inherently incorporates individual and contextual variability within each sequential level of analysis.
In addition, the regression models adjusted for self-reported-time of awakening, four time of the day dummy coded variables measuring cortisol concentration at occasion 2 (30 minutes after awakening), at occasion 3 (14:00 hours), at occasion 4 (16:00 hours), and at occasion 5 (bedtime), as well as two dummy coded variables indexing cortisol concentrations for workday 1 and workday 2 compared to the non-workday. Statistical analyses were also controlled for sex, age, season of sampling, smoking, alcohol, physical activities, psychotropic drug use, physical health problems, and BMI. The model parameters were estimated by the restricted iterative generalized least-squares method (RIGLS) using MLwiN Statistical Software version 2.26 (Center for Multilevel Modeling, University of Bristol, UK). To reduce the asymmetrical distribution and improve the convergence of the estimation algorithm, cortisol concentrations in ug/dl were multiplied by 100 and log-transformed (natural logarithm). The main effect model was first estimated, followed by a series of interaction tests between mental health measures and time of the day. Finally interactions with sex were estimated to assess potential sex moderating effects. The significance of individual regression coefficients was evaluated using a bilateral Z test, and the probability of rejection of the null hypothesis was set at P<0.05. The random coefficients were tested with a halved P-value (28). The joint contribution of the variables was assessed by means of a likelihood ratio test that followed a χ2 distribution with the degrees of freedom (df) equal to the number of additional parameters in the model. Interactions were tested using χ2 with rejection of the null hypothesis at P<0.05.
Finally in separate analyses of an illustrative nature, line-plots and error-bars of the relations between cortisol concentrations at each occasion and mental health symptoms evaluated by questionnaires were graphed using regression coefficients obtained from the multilevel regression analysis. Scores were then reverted back into original ug/dl units for the sake of comparison. We used standard cut-off points for psychological distress and depression; severe psychological distress symptoms corresponded to a score of 8 on the GHQ-12 (29), whereas severe depressive symptoms were based on a cut-off point of 29 on the BDI-21 (24). For burnout, there is no consensus regarding cut-off points; however, using a meaningful cut-off point (average score of 4), symptoms were classified according to minimal and severe burnout symptoms. The reader will recall that a score of ≥4 represents self-reported burnout symptoms ≥1 week.
Results
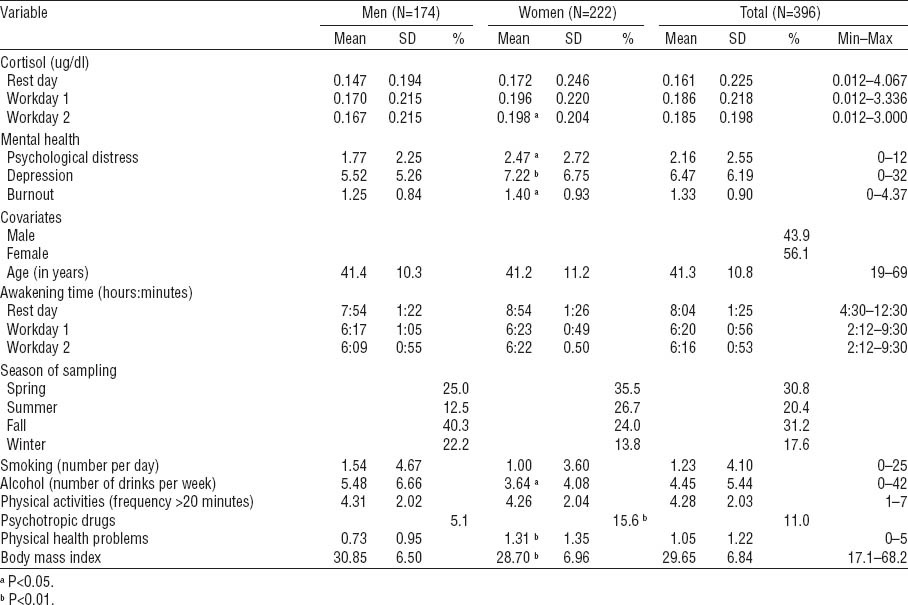
Table 1 reports raw cortisol concentrations and the characteristics of the study sample. Compared to men, women showed higher cortisol concentration on workday 2, psychological distress, depressive and burnout symptoms, psychotropic drug use, and health conditions, but a lower number of alcoholic drinks per week and a lower BMI.
Multivariate analysis
Psychological distress was correlated with depressive (r=0.69, P<0.01) and burnout (r=0.48, P<0.01) symptoms; depressive symptoms were correlated with burnout symptoms (r=0.63, P<0.01). A first series of multilevel regression models estimated the main effects of mental health measurements on cortisol concentrations adjusted for awakening time, occasions (30 minutes after awakening, 14:00 and 16:00 hours, and bedtime), work and non-work days, sex, age, season of sampling, alcohol use, physical activities, psychotropic drugs use, physical health, and BMI. Results of main effects analysis assessing global cortisol concentrations – that is, the grand mean of samples without regard for diurnal time points – revealed no significant associations with psychological distress (χ2=0.29, df=1, P=0.590), and depressive (χ2=0.01, df=1, P=0.920) and burnout (χ2=0.46, df=1, P=0.498) symptoms.
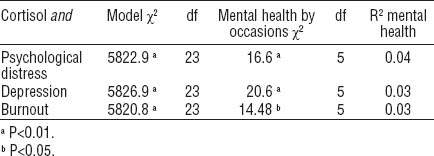
The next series of models estimated the extent to which mental health measurements interacted with work/non-work days and occasions for specific diurnal cortisol time points. No significant interactions were found with days and psychological distress (χ2=3.97, df=1, P=0.137), and depressive (χ2=1.38, df=1, P=0.502) and burnout (χ2=1.53, df=1, P=0.466) symptoms. However, mental health symptoms interacted with occasions (time of day; results are presented in tables 2 and 3).
Table 2
Results of multilevel regression modelling of cortisol concentrations in ln[(ug/dl)×100] for psychological distress, depression, and burnout. [SE=standard error]
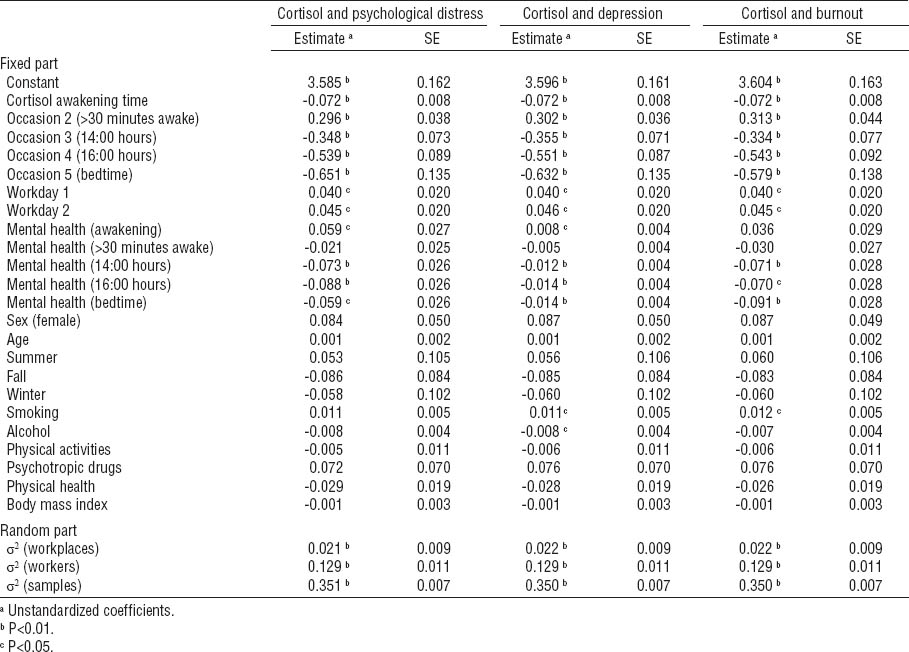
In analyses centered on diurnal cortisol time points, results of the fixed part of the three models show that cortisol concentrations increase 30 minutes after awakening and decline for the rest of the day. The results also show an increase in cortisol concentrations on working days in contrast to the rest day.
Cortisol concentrations across the day were associated with mental health symptoms. For psychological distress, increased scores were positively associated with cortisol concentrations at awakening and negatively associated with cortisol concentrations at 14:00 and 16:00 hours and bedtime. Identically for depression, scores were positively associated with cortisol concentrations at awakening, and negatively associated with cortisol concentrations at 14:00 and 16:00 hours and bedtime. As for burnout, scores were only negatively associated with cortisol concentrations at 14:00 and 16:00 hours and bedtime, but not correlated with morning cortisol concentrations. No associations were detected for psychological distress and depressive and burnout symptoms with respect to cortisol concentrations 30 minutes after awakening.
The random part of the models revealed that overall cortisol concentrations vary significantly between samples, workers, and workplaces. More than 4% of cortisol concentrations variation is between workplaces. Overall, however, the effect sizes of mental health indicators on cortisol concentration were rather low. Indeed, psychological distress accounts for 4% of the variance in cortisol secretions, while depressive or burnout symptoms explain only 3% each. In terms of covariates, smoking was positively correlated with cortisol concentrations in association with symptoms of depression and burnout, while alcohol consumption was negatively correlated with cortisol concentrations in association with depressive symptoms. No other covariates attained statistical significance.
The last series of models estimated the extent to which sex interacts with mental health symptoms and time of the day. No significant interaction effects were found for psychological distress (χ2=4.75, df=5, P=0.445) and depressive (χ2=1.90, df=5, P=0.863) and burnout (χ2=4.01, df=5, P=0.548) symptoms, revealing that sex did not moderate our study findings.
Based on information derived from the multilevel regression models, figure 1 was generated to show the relationship between severe symptoms of psychological, depression, and burnout, and variation of cortisol concentrations across the five sampling occasions.
Figure 1
Multilevel regression predicted cortisol profiles according to severe mental health symptoms.
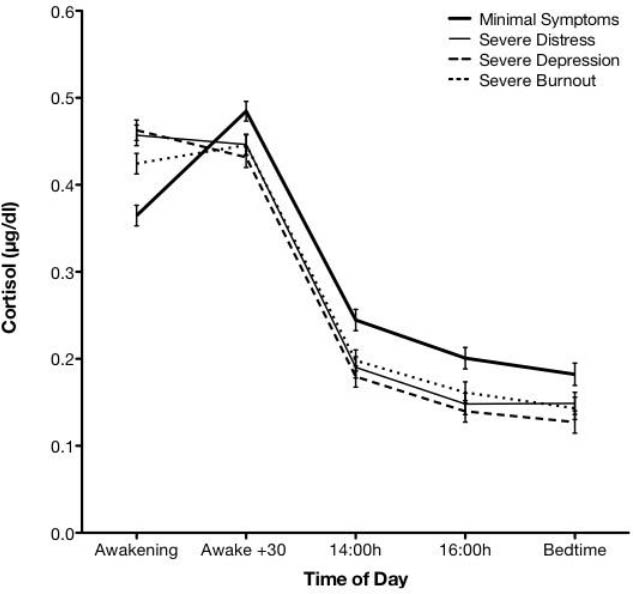
As depicted in figure 1, all severe mental health symptomatologies are associated with higher cortisol concentrations at awakening compared to minimal symptoms. In addition, cortisol concentrations at awakening are significantly higher for psychological distress and depression compared to burnout. In contrast to the sharp increase in cortisol concentrations 30 minutes after awakening, all mental health symptoms demonstrated minimal increases from awakening to 30 minutes. It is important to note, however, that cortisol concentrations did not significantly differ according to symptom severities for the 30 minutes post-awakening time point. By contrast, marked differences re-emerged for the 14:00 and 16:00 hours and bedtime time points, whereby all severe mental health symptoms are associated with lower cortisol concentrations compared to minimal symptoms.
In summary, figure 1 shows that there are no significant differences in patterns of cortisol concentrations in relation to specific types of severe mental health symptoms, except at awakening whereby burnout cortisol concentrations are lower than those of psychological distress and depression, but still higher than those with minimal symptoms. While indistinguishable from each other, all severe mental health symptomatologies cortisol concentrations were lower throughout the afternoon and evening in comparison to minimal symptomatologies.
Discussion
This study aimed to assess the comparative associations of psychological distress and depressive and burnout symptoms with diurnal cortisol profiles among day-shift workers. By integrating time of the day for working and non-working days and after adjustment for various confounders, we found significant associations between cortisol concentrations across the time of day and self-reported psychological distress and depressive and burnout symptoms. However, the magnitude of these associations was relatively small, ranging from 3–4% of the explained variance. Cortisol variation was additionally found between workplaces consistent with the notion that environmental differences influence stress physiology. Finally, associations were not moderated by work/non-work days or sex.
In terms of overall diurnal rhythmicity, cortisol concentrations increased from awakening to 30 minutes after awakening indicative of a functional CAR, declining steadily thereafter until bedtime consistent with previous reports (13, 30). Cortisol concentrations were, on average, elevated during working compared to non-working days, which is again concordant with previous studies (11, 31); however, unlike previous reports, associations did not differ according to one’s sex. Our results suggest that working days generally demand higher levels of physical and mental activity that generate increased HPA-axis output irrespective of one’s sex when multiple covariates are accounted for. While this trajectory linking psychological factors to cortisol profiles is logical, it must also be acknowledged that the opposite pathway may reciprocally modulate effects, such that increased cortisol concentrations prompt elevated distress and psychiatric symptoms. Only prospective study designs can address this.
Nevertheless, our cross-sectional results revealed that as symptoms of psychological distress and depression increased, awakening cortisol concentrations were higher than among those with minimal symptoms. This would suggest that as workers become increasingly distressed and depressed, the higher their manifested cortisol concentrations at awakening. This is reminiscent of reports suggesting that depression is a hypercortisolemic condition (32, 33) as well as Chida and Steptoe’s meta-analysis (13) that found hypercortisolemic CAR among distressed and depressed participants and hypocortisolemic CAR among fatigued and burnt-out participants. It is interesting to note that burnout symptomatology was related to lower cortisol levels than distress and depression at awakening in the current sample (see figure 1), consistent with a slightly blunted CAR in comparison to distress and depression, but still higher than minimal symptoms. Notwithstanding, awakening levels in the current study provide insight only into the supposed starting time of the CAR, and not the CAR in its entirety. Our results might be better explained by an earlier set-point for CAR initiation among workers with elevated symptomatologies. This would imply that it is not necessarily that the CAR is hyperactive or hypoactive, but that it starts earlier on while workers are still asleep and/or slowly awakening. This proposal will require continued investigations using complementary methods that can assess circadian patterns, sleep hygiene, and cognitive processes that were not assessed in the current study.
Beyond awakening elevations among distressed and depressed workers, our study does not support the overall notion of a hyperactive CAR given that cortisol appeared blunted 30 minutes after awakening in comparison to a healthy CAR. While cortisol clearly increased 30 minutes after awakening for minimal symptoms of mental health (figure 1), such a profile cannot be observed with elevated symptoms of psychological distress, depression, and burnout, suggesting that the CAR might instead be down-regulated under conditions of severe distress and psychiatric symptomatologies. This suggests that mental health symptoms might weaken the usual physiological “kick” needed to begin daily activities. This may seem counter-intuitive given that distress and depression were associated with elevated awakening levels. If the CAR did indeed initiate earlier as we propose, than it would be normal to have comparatively lower levels 30 minutes after awakening since the CAR would have been activated for a longer period of time. Such diversity of responsivity in morning samples alone speaks to the importance of multiple sampling time points in large-scale studies of HPA-axis functioning (34) as well as the application of trans-disciplinary approaches that can triangulate methods.
In contrast to morning cortisol profiles, severe mental health symptoms were associated with sharper decreases in cortisol secretions compared to minimal symptoms observed at 14:00 and 16:00 hours and bedtime. Apart from awakening cortisol concentrations and the CAR (13, 35), it is unknown whether afternoon and evening cortisol profiles differ, especially in relation to overlapping psychiatric conditions like depression and burnout (36–38). Beyond psychometric delineation that is complemented using biometrics, the consistently lower cortisol concentrations among severe distress and depressive and burnout symptoms in comparison to minimal symptoms suggest a down-regulation of the HPA-axis. In visualizing the overall diurnal cortisol rhythm depicted in figure 1, it appears that cortisol concentrations are recalibrated among severe symptomatologies in a manner that appears sluggish and less variable in comparison to low symptoms. Clearly, longitudinal research is needed to clarify daytime differences in cortisol as a function of psychopathology among healthy and unhealthy workers.
This study has a number of limitations. First, the survey design was cross-sectional and cannot infer the directionality of psychological and biological associations. Second, the enrolment of volunteers in this study may have caused a selection bias. Third, even though participants completed logbooks of saliva collection times, it would have been preferable to monitor compliance using electronic monitoring technologies (39). Hence, there is some variation in the collection time points for saliva samples due the fact that participants sometimes forgot to collect samples or delays occurred for other reasons. Variations due to lack of compliance thus engender measurement error that are difficult to evaluate; however, these errors are common in field studies even when strict protocols are maintained.
Lastly, participants provided scores for psychological distress, depression, and burnout that are all correlated to each other. In relation to cortisol concentrations, the relative contribution of each psychometric is therefore not physiologically distinct since workers experienced each mental health symptom to some degree. The objective of this study was not, however, to decipher the cortisol profiles of specific psychopathologies, but rather to simply ascertain whether subjective psychometrics correspond with objective physiological parameters. In order to distinguish psychometric profiles in relation to biological profiles, future studies would have to recruit contrasting patient populations that might ultimately be associated with different cortisol profiles over the course of the disease over time. Future research might consider assessing cortisol from hair samples to study the relationship between mental health and cortisol retrospectively. Hair cortisol measures HPA-axis excretion months earlier, thus representing long-term cortisol excretion and not short-term concentrations as currently assessed from saliva samples (40).
Beyond these limitations, this study suggests that distinct diurnal HPA-axis functioning is associated with higher levels of subjective reports of psychological distress and depressive and burnout symptoms. Although effect sizes were relatively small in explaining cortisol concentrations variations, self-reported evaluation of mental health may represent a valid and a reliable tool to capture particularities not only in mental health functioning, but also as a means to infer possible HPA-axis malfunctioning. Given that specific psychiatric symptoms correspond with similar yet subtly distinct cortisol profiles throughout the day, it will be important for future researchers to delineate the clinical significance of even the smallest variation that potentially signals impending mental illness. Moreover, the overlap among subjective and objective indices of distress points to the importance of triangulating methods when attempting to understand complex, multifaceted phenomena like workplace stress and the prediction of disease outcomes.
In conclusion, our findings suggest that awakening, afternoon, and bedtime cortisol concentrations are reflected in mental health psychometrics measured using the GHQ-12, BDI-21, and MBIGS-16 questionnaires. They support the notion that subjective psychometrics correspond with objective biometrics among healthy workers. We believe that the approach carried out in this study is particularly useful in understanding how psychological and physiological functioning are associated, which could eventually lead to further refinement of mental health questionnaires routinely used in epidemiologic, population health, and occupational health studies. More research is thus needed to identify better cut-off points on available self-reported mental health questionnaires and their correspondence to specific cortisol profiles over time. Such cut-off point determination will help scientists and practitioners prevent and/or intervene before worker symptoms reach unsustainable levels that ultimately increase the odds of absenteeism and the development of stress-related conditions, such as psychological distress, depression, and burnout.