Hard end points such as mortality, morbidity, and target vessel revascularization rates are well documented after percutaneous coronary intervention (PCI), but relatively scarce data exist on occupational health outcomes. Among patients of working age, permanent disability pension has major socioeconomic consequences for both the individual and the society. Nevertheless, little is known about the rate and reasons for permanent work disability (PWD).
In this pre-specified analysis of the CRAGS (Coronary aRtery diseAse in younG adultS) registry we sought to assess the role of periprocedural factors related to PCI in the incidence, indications, and determinants of permanent disability pension in long-term follow-up of ≤50-year-old patients who have undergone PCI (1–3).
Methods
This study is part of a larger multicenter collaboration designed to describe outcomes among ≤50-year-old patients revascularized for coronary artery disease (CRAGS). This substudy comprised all 1003 consecutive PCI ≤50-year-old patients who underwent PCI at three university hospitals (Oulu, Tampere and Turku) and one central hospital (Vaasa) in Finland during 2002–2012. Invasive treatment of coronary disease is mainly centralized and the participating hospitals are the treating hospitals within their geographical catchment area.
The study complies with the Declaration of Helsinki, and the locally appointed ethics committees of participating hospital have approved the research protocol.
Data on PWD pension (starting date, primary and secondary diagnosis) were acquired from the Finnish Centre for Pensions, which governs the statutory pension security in Finland, and which in turn consists of a defined benefit earnings-related pension that accrues from work as well as residence-based national pension and guarantee pension that ensure minimum security. The statutory pension system in Finland consists of two main parts ─ earnings-related and residence-based national pensions. The earnings-related pension, managed by various providers who belong to the Finnish Centre for Pensions, covers the entire workforce, including the self-employed. None of the patients were eligible for old-age pension during follow-up. Ninety-three patients on PWD already prior to index procedure were excluded from further analysis. Periprocedural data and baseline comorbidities along with data on post procedural morbidity were acquired from local hospital registries which enabled complete follow up as treatment of the events of interest (cardiac- and cerebrovascular) are centralized. Data on date and mode of death was acquired from the Finnish national registry, Statistics Finland.
Data on the number of insured and the incidence and category (cardiovascular, musculoskeletal and psychiatric) of PWD in each age group in the general population between 2007–2011 was obtained from the Finnish Center for Pensions. Statistics from a group comprised of 45-year-old insured patients were used for comparison with the study population (mean age 45 years). Major adverse cardiac and cerebrovascular event (MACCE) is defined as a composite of myocardial infarction (MI), repeat revascularization and stroke. Death was not included as MACCE was evaluated as a factor influencing PWD.
The main outcomes of interest were PWD defined as grant of disability pension during follow-up and predictors thereof. Secondary measures were predictors of cardiac disability defined as PWD with a cardiac diagnosis. Statistical analysis was performed with SPSS Statistics version 22.0 (IBM, Armonk, NY, USA) and R version 2.15.3. Categorical variables are reported as counts and percentages, continuous variables as mean and standard deviation (SD) or median and interquartile range (IQR). Fisher’s exact test, Chi square, independent samples T-test and Kaplan-Meier were used for univariate analysis. Cox proportional hazards models were employed to identify predictors of permanent disability by including clinically important variables with a P-value <0.10 on univariable analysis into the multivariable models.
Results
By the end of a median follow-up of 41 (SD 31) months, there were 103 cases of PWD (11.3% of the study population).
Time to grant of disability pension was 34 (SD 29) months overall and 29 (SD 27) for cardiac PWD versus 42 (SD 32) for other types of PWD (P<0.001).
Primary diagnoses for PWD were cardiac in 43/103 (41.7%), psychiatric in 15/103 (14.6%), and musculoskeletal in 16/103 (15.5%) patients. Diagnoses for PWD in the general population were cardiac in 5.7%, psychiatric in 40.1%, and musculoskeletal in 24.4%. In the study population, 11/15 primary psychiatric diagnoses were depression and 19/103 (18.4%) of patients had depression as a primary or secondary diagnosis for PWD.
For the 93 patients who were excluded from analysis for preprocedural PWD, primary diagnoses for PWD were: cardiac for 12 (12.9%), musculoskeletal for 18 (19.4%), and psychiatric for 26 (28%) patients.
Baseline variables and outcomes for patients with and without post procedural PWD are detailed in table 1. Patients who developed PWD were slightly older and had a higher baseline comorbidity burden compared to patients without later disability.
Table 1
Baseline and postprocedural characteristics of patients <50 years old with or without permanent disability compensation after percutaneous coronary intervention. [AP=angina pectoris; ASA=acetosalicylic acid; AT2=angiotensin 2; CABG=coronary artery bypass grafting; CAD=coronary artery disease eGFR=estimated glomerular filtration rate (MDRD formula); LAD=left anterior descending artery; LCX=left circumflex artery; LVEF=left ventricular ejection fraction; MI=myocardial infarction; MSK=musculoskeletal; NSTEMI=non ST-elevation MI; PCI=percutaneous coronary intervention, RCA=right coronary artery; STEMI=ST-elevation MI].

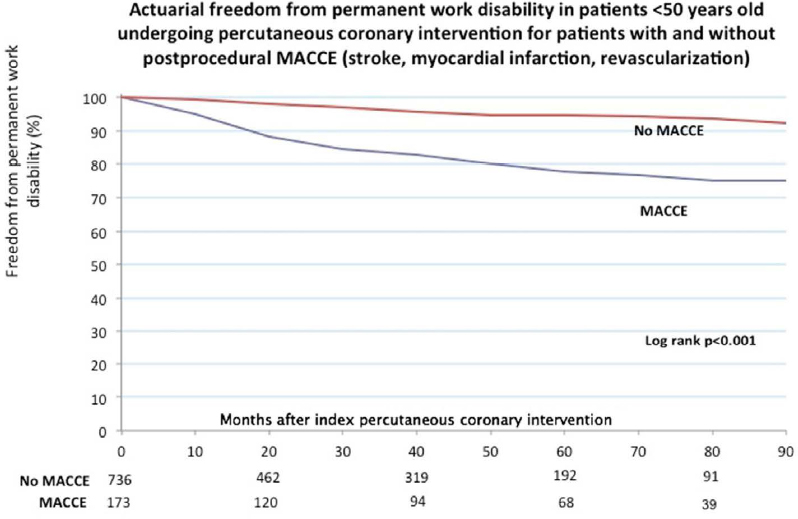
A total of 24/73 (32.9%) patients with a recurrent MI, 36/149 (24.2%) patients with a repeat revascularization procedure, and 6/12 (50%) patients who experienced a post procedural stroke were on PWD by end of follow-up. Kaplan-Meier estimates of freedom from PWD for patients with and without post procedural MACCE are represented in figure 1. Kaplan-Meier estimates for overall freedom from PWD were 88.0% at 4 years and 81% at 7 years. Actuarial freedom from PWD at 4 years in the general population aged 45 years old was 97.2%.
Figure 1
CART (Classification and regression tree analysis) for discriminators of permanent work disability following percutaneous coronary intervention (PCI) among patients ≤50 years old.

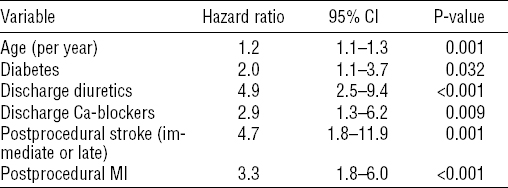
Independent predictors of PWD on Cox regression are detailed in table 2. PCI on left anterior descending branch was an independent protective factor against PWD [hazard ratio (HR) 0.6, 95% confidence interval (95% CI) 0.4–0.9, P=0.019].
Table 2
Predictors of permanent disability after percutaneous coronary intervention (PCI) among patients <50 years old. [95% CI=95% confidence interval; MI=myocardial infarction.]
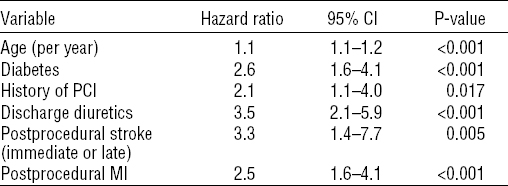
Patients with PWD for cardiac diagnosis were older [47 (SD 3) versus 45 (SD 5) years, P=0.001], more often had dyslipidemia (38.3% versus 25.7%, P=0.03), hypertension (48.3% versus 28.8%, P=0.001), and diabetes (26.7% versus 11.3%, P<0.001). Kaplan-Meier freedom from cardiac PWD was 89.6% at 7 years. Independent predictors of cardiac PWD were age, diabetes, discharge diuretics, discharge calcium-channel blockers, post procedural stroke, and post procedural myocardial infarction (table 3.). PCI on left anterior descending artery (LAD) emerged as an independent protective factor against cardiac PWD (HR 0.4, 95%CI 0.2–0.7, P=0.002).
Discussion
The present study provides details on a socioeconomically important subpopulation of ≤50-year-old patients referred for PCI. This is a patient group with a potential for longevity and, as an age group, in the prime of their input in the workplace. Previous studies have reported that 64–87% of young patients return to work within 3–15 months after PCI (4–7); many of the studies, however, are from the 80s and 90s making comparison with contemporary PCI techniques and modern employment policies very difficult. Furthermore, the studies do not describe long-term working capacity after PCI. Comparisons are rendered difficult also by the fact that the present registry does not contain data on employment status, but rather whether or not the patient has been deemed capable of work. The excellent results survival-wise (1, 8) in the present registry are offset by a high cumulative incidence at 7 years follow-up of PWD (19.0%), and 58.3% of patients with PWD had a cardiac diagnoses as the primary indication for disability pension. The patients with cardiac PWD left the work force one year earlier than those with non-cardiac indication for pension. The 4-year freedom for patients (median age 45 years) was 88.0% compared with 97.3% in the general population aged 45 years based on statistics from the Finnish Centre for Pensions.
Important predictor baseline values of both overall and specifically cardiac disability were increasing age, diabetes, and previous coronary interventions which are known predictors of MACCE among young patients undergoing PCI (8, 9).
The predictive role of diabetes is not surprising, since diabetics in general have a worse prognosis after revascularization than non-diabetics, and diabetes by itself also negatively influences working ability (10–13). Post procedural adverse events were associated with permanent disability both in univariate and multivariate analysis. A possible place for improvement might be a more complete approach to revascularization; patients with permanent disability pension more often had incomplete revascularization, which according to the literature is a risk factor for adverse outcomes and it is possible that a more aggressive approach towards a complete revascularization in these young patients might reduce the need for disability pension (14, 15).
On the other hand, most of the post-PCI revascularization procedures in this registry were performed due to disease progression suggesting that shortcomings in secondary prevention play a larger role (3). Based on the results, <50-year-old patients have an almost universal optimal medication at the time of hospital discharge from PCI. However, in general, 25–60% of patients discontinue optimal discharge medication one year after PCI (16–18). Moreover, young age in itself is a known predictor of both adverse events as well as poor adherence to secondary prevention (19, 20). Patients in this study also had a noteworthy incidence (27.2% of patients on PWD) of psychiatric morbidity leading or contributing to PWD; psychiatric symptomatology, even if not directly leading to disability, causes poor adherence to medication (18, 21), which in turn can affect outcomes and cardiac health entailing impaired working health. It has also been shown that improvement in depressive symptoms leads to better adherence to secondary prevention among cardiac patients (22, 23). Serious mental illness itself is also a well described risk factor for cardiovascular morbidity and mortality but even less dramatic psychiatric morbidity such as depression incurs an elevated risk of cardiovascular events (24–26). Even though the percentage of actual psychiatric diagnoses in the present study was in fact lower than in the PWD cases of the general population a psychosocial burden is likely in young patients undergoing PCI. Alluding to this for example is the high prevalence of smoking of about 70% which is distinctly higher than in the general Finnish population (Finnish National Institute for Health and Healthcare 2013 Tobacco Statistics) and the high prevalence of smoking is typical for lower socioeconomic demographics (3, 27).
Figure 2
Freedom from permanent work disability among patients ≤50 years old undergoing percutaneous coronary intervention (PCI). [MACCE=major adverse cerebrovascular and cardiac event (stroke, revascularization, acute myocardial infarction).]
Cardiac rehabilitation, in addition to improving functional status cost-effectively (28, 29), has a protective effect against cardiac events further preserving function and working ability (30, 31). Improving participation in cardiac rehabilitation programs can be difficult, but attendance can be improved by addressing system- and patient-level barriers (32) while physical barriers other than coronary disease, such as those prevalent in the current study population, can still prevent attendance (33). It is also noteworthy that the deleterious effects of incomplete revascularization can also be mitigated by rehabilitation, and cardiac rehabilitation improves adherence to secondary prevention (34). Early and active collaboration with occupational healthcare physicians and rehabilitation could be potential targets for improving later working capacity in young patients.
Gender did not independently influence the risk of disability unlike the earlier literature on disability in general and after PCI (35–37). Remarkably even the higher baseline disease burden among women in the present study (data not shown) did not alter the incidence of PWD.
In a previous extensive study from Sweden – a country with a healthcare and pension system very similar to Finland – PWD rates were higher compared to our study (36). Authors reported almost a two-fold incidence (29.3%) of disability pension compared with this study during a 5-year follow-up. The patients in the Swedish study, however, were older with over 80% being ≥50 years at index procedure. Although Zetterström et al’s study focused on psychosocial factors of PWD (36), which are also inextricably present in this study, it also highlighted the impact of disease burden at baseline on later occupational status. While psychosocial factors play a large role in future PWD, socioeconomic factors alone typically do not elicit rehabilitative and occupational healthcare measures. The combination, at least, of socioeconomic disadvantage, psychiatric symptomatology and cardiovascular disease should act as a red flag entailing supportive measures to uphold working capacity.
Limitations
These findings need to be interpreted with caution. The main limitation of this study is its base in one healthcare and health insurance system; although this makes direct numerical applicability into other systems unreliable it does give a good overview of the comorbidities and predictors of disability in young PCI patients. This study also does not assess employment status, only permanent disability. The actual percentage of patients out of the workforce can thus be even higher. Also missing are other sociodemographic data such as occupational and educational background which were not recorded in the registry as the main focus of the present study was on periprocedural factors and their association with disability. The retrospective nature of the study is also a limitation, but a prospective study on this issue would hardly be feasible because of the limited number of young patients treated yearly in each center. In addition, the present registry has all the inherent limitations of an observational study, including individual risk-based decision-making in treatment choices. Compliance data on secondary preventive medication was not available.
Concluding remarks
Patients <50 years old undergoing PCI are at high risk of subsequent PWD pension. Post procedural adverse events such as MI, repeat revascularization, and cerebrovascular events are the major predictors of later disability. A more active approach to secondary prevention, cardiac rehabilitation and occupational health is needed among young patients after PCI.