High-level disinfectants (HLD) are used throughout the healthcare industry to disinfect reusable medical devices chemically and control and prevent healthcare-associated infections. HLD are not the same as most common household disinfectants (eg, surface sprays containing quaternary ammonium compound disinfectants or bleach) and are differentiated by their ability to destroy or eliminate all forms of microbial life, except large numbers of bacterial spores, rather than just a specific type (1).
In an Environmental Working Group and American Nurses Association survey of over 1500 nurses, >50% of nurses reported any on-the-job exposure to HLD, 20% reported regular exposure for ≥10 years, and 16% reported exposure while pregnant (2). Despite the widespread use of HLD, their reproductive health consequences remain mostly unclear (3–5). Experimental studies have shown that female laboratory animals exposed to HLD before and during early pregnancy had reduced fertility (6) and experienced adverse reproductive outcomes, including embryo toxicity, fetal death, reduction of fetal weight, and congenital malformations (7–9); however not all animal studies have found these detrimental reproductive effects (10, 11). In humans, studies have shown that women exposed to chemical disinfectants and sterilizing agents during pregnancy have a higher risk of miscarriage and preterm birth (12–16).
To date, no study has examined the relation between HLD and fecundity. Furthermore, no studies on exposure to HLD and reproductive outcomes have collected information on the use of personal protective equipment or engineering controls, which could be an important effect mediator. Therefore, our objective was to (i) evaluate the relationship between occupational use of HLD and fecundity among female nurses and (ii) assess whether protective equipment modified this relation.
Methods
Study population
The Nurses’ Health Study 3 (NHS3) is an ongoing internet-based cohort study of female nurses in the United States and Canada. To be eligible for the study, women had to be either a registered nurse, licensed practical/vocational nurse or nursing student and born on or after 1 January 1965. As of September 2014, 38 016 women had joined the study and 26 693 women had completed ≥1 follow-up questionnaire, forming the base population for our analysis. Every six months questionnaires are sent to participants to update lifestyle and medical characteristics. The response rate for the second questionnaire is currently at 72%; for women who have completed ≥2 questionnaires, subsequent response rates exceed 80%. Women were eligible for this current analysis if they reported working as a nurse on their baseline questionnaire and on any of the subsequent questionnaires that they were trying to get pregnant (N=1757). We excluded women who reported that they were postmenopausal (N=15) or were missing information on duration of ongoing pregnancy attempt (N=3). After these exclusions, 1739 women were available for analysis. The Institutional Review Boards of the Brigham and Women’s Hospital (Boston, Massachusetts) and the National Institute for Occupational Safety and Health (Cincinnati, Ohio) approved the study. Completion of the web-based questionnaires implied informed consent.
Exposure assessment
The baseline questionnaire collects information about work schedule, physical aspects of work, and select occupational exposures including radiation, aerosolized drugs, antineoplastic agents, HLD, and anesthetic gases. The questions on HLD use begins with the following gateway question, “In your career, have you ever used disinfectants to disinfect medical instruments, devices or supplies (such as endoscopes, thermometers or other items which cannot be sterilized) by either manual or automatic methods? (This does not include the cleaning of countertops or other surfaces)”. The questionnaire then lists several examples of disinfectants. If a woman indicates she has been exposed to HLD, she is then asked how long during her career she has been using HLD, how much total time she spent handling disinfecting agents over an average week in the past month, and to specify all agents she used in the past month from the following list: glutaraldehyde (eg, Cidex®, ColdSport®, Endocide®, Glutacide®, Hospex®, Metricide®, Sporicidin®, Wavicide®), ortho-phthalaldehyde (eg, Cidex OPA®), peracetic acid (eg, Steris® system), hydrogen peroxide (eg, Accell®, Optim®), ethylene oxide, formaldehyde, or other. Women reporting use of HLD in the past month were then asked to report how often they used each of the following personal protective equipment or engineering control [collectively referred to as protective equipment (PE) for this paper]: a disinfection system with dedicated local exhaust ventilation (overhead hood, exhaust fan), a water resistant gown or outer garment, protective gloves, eye protection (safety glasses, goggles, or face shield), and respiratory protection (not including a surgical mask). Response categories for use of the PE were “always”, “sometimes”, and “never”.
Outcome assessment
Women who report they are actively trying to get pregnant are asked to report the current duration of their ongoing pregnancy attempt. Specifically, they are asked: “For how many months have you been actively trying to get pregnant?” Categories for response include: ≤1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 months, 1–2 years, and ≥3 years. We took a woman’s first report of ongoing pregnancy attempt after the baseline questionnaire as her outcome. As such, the majority of current durations were reported on questionnaire 2 (65%) followed by questionnaire 3 (21%) and questionnaire 4 (14%). Validity of self-report of duration of pregnancy attempt has not been assessed in this population; however, it is considered a valid methodology for assessment of fecundity among pregnancy planners (17, 18).
Covariate assessment
Information on potential confounding variables was assessed on the baseline questionnaire, including age, race/ethnicity, height, weight, lifetime pregnancy history, smoking history, and marital status. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. In a previous validation study, self-reported weight was highly correlated with weight measured by a technician among a similar group of nurses (r=0.97) (19). Menstrual cycle characteristics including current regularity and length of a usual menstrual cycle are reported on the first follow-up questionnaire. Participants provided the current regularity of their menstrual cycles in the following categories: “very regular (+/- 3 days), regular, usually irregular, or always irregular”. For analysis, we compared “regular” (very regular or regular) to “irregular” (usually or always irregular). Menstrual cycle length was reported in the following categories: <21, 21–25, 26–31, 32–39, 40–50, and >50 days or too irregular to estimate. For analysis, we defined a normal length menstrual cycle as lasting 21–39 days and all other categories were considered abnormal length. We also categorized menstrual cycle length into short (≤25 days), normal (26–31 days), long (32–50 days), and >50 days (only if a woman reported usually or always regular menstrual cycles).
Statistical analysis
For the main analyses, women were classified into three mutually exclusive categories of HLD use: “never”, “used prior to baseline”, and “current use at baseline”. We tested for differences in demographic, lifestyle, and reproductive characteristics across categories of HLD use using chi-square tests (or Fisher’s exact test where appropriate) for categorical variables and Scheffe tests for continuous variables. Other metrics of HLD use such as frequency and duration of use were categorized according to the distribution in our cohort. For instance, due to the low frequency of current HLD use, women working 1–5 (N=71), 6–20 (N=9), and 21–40 (N=0) hours per week were combined into a single category of ≥1 hour/week.
To analyze the association between HLD use and fecundity, we used a current duration approach which uses information collected in a cross-sectional fashion on current duration of ongoing pregnancy attempt to make inferences about actually realized waiting times to pregnancy (20). Other studies have utilized this approach to estimate the national prevalence of infertility (21, 22) and the association of environmental factors on fecundity (23). We have also previously used this approach to evaluate work schedule and body weight changes in relation to fecundity (24, 25). Since couples who have long durations of attempting pregnancy are overrepresented in the current duration approach, appropriate statistical models are used to account for this length-biased sampling. The current duration approach and more generally backward recurrence time survival methods allow us to infer the relationship of characteristics to the (unobserved) total duration of pregnancy attempt by using the (observed) current duration of attempt via accelerated failure time models (20). Based on previous research, we chose an accelerated failure time model with log normal distribution to estimate the time ratios (TR) and 95% confidence intervals (95% CI) (23). The TR correspond to exp([beta]) and can be interpreted as the ratios of the median values of the duration of pregnancy attempts between the compared groups. Tests for linear trend across categories were conducted by using the median value in each category as a continuous variable. In addition to unadjusted models, multivariable models were adjusted for a priori selected demographic and occupational variables. These included current age, BMI, smoking status, marital status, race, hours per week of nursing work, frequency of lifting or moving a heavy load, and current exposure to radiation, antineoplastics, anesthesia gas, and aerosol drugs. We ran models with and without adjusting for pregnancy history, as adjustment for reproductive history might lead to over adjustment if ongoing work characteristics are related to the inability to get pregnant, which could manifest as nulligravidy (26, 27). The directed acyclic graph we used to visualize the possible confounding structure of our association is shown in supplemental figure S1 (www.sjweh.fi/index.php?page=data-repository).
To address effect modification by PE use, we cross-classified currently exposed women into exposure categories based on “always or sometimes use” and “never use” of specific types of PE. The combination of “always” and “sometimes” use into one category was based on the low frequency of responses in these two categories. Differences in the effect of current HLD use on fecundity among users versus never users of PE were tested using a Wald chi-square test. As a sub-analysis, we evaluated whether baseline demographic and occupation characteristics were associated with PE use among women currently exposed to HLD using chi-square tests (or Fisher’s exact test where appropriate) for categorical variables and Kruskal-Wallis tests for continuous variables.
We also conducted several sensitivity analyses to assess the robustness of our study design and analysis. The first sensitivity analysis restricted our population to women with current durations of pregnancy attempt reported within two years of the baseline exposure assessment to minimize exposure misclassification. The second sensitivity analysis truncated current durations of pregnancy attempt >12 months to 12 months to explore the potential influence of the very long pregnancy attempts. Finally, the third sensitivity analysis used a generalized gamma outcome distribution (instead of a log-normal distribution) to test our assumptions regarding the fit of our outcome distribution. Effect modification by age (<37 versus ≥37 years), BMI (<25 versus ≥25 kg/m2), smoking status (never versus ever smokers), and gravidity (nulligravid versus gravid), four of the best characterized factors associated with fecundity, was tested using a cross-product term in the final multivariate model. SAS statistical software, version 9.3 (SAS Institute, Cary, NC, USA) and a significance level of P<0.05 was used for all analyses.
Results
Thirty one percent of the nurses in our cohort reported ever use of HLD with 12% reporting exposure prior to baseline and 19% reporting current exposure at baseline. On average, women with exposure to HLD prior to baseline were older, heavier, less likely to be nulligravid, nulliparous, and move or lift heavy loads at work, and more likely to work day-only shifts compared to women never exposed to HLD (table 1). Women with current exposure to HLD at baseline were, on average, less likely to work <20 hours per week and more likely to move or lift heavy loads at work and be currently exposed to antineoplastic drugs, radiation, aerosol agents, and anesthetic gas compared to women never exposed to HLD.
Table 1
Age-standardized baseline demographic and occupational characteristics by use of high level disinfectants among women attempting pregnancy in the Nurses’ Health Study 3 (N=1739). All values are standardized to the age distribution of the study population.[BMI=body mass index; BSN=Bachelors of science in nursing; LPN=licensed practical nurse; RN=registered nurse; SD=standard deviation].
| Use of high-level disinfectants | ||||
|---|---|---|---|---|
|
|
||||
| Never (N=1202) a | Yes, prior to baseline (N=215) b | Yes, at baseline (N=322) c | ||
|
|
|
|
||
| % | % | % | ||
| Race | ||||
| White | 91.2 | 89.7 | 91.1 | |
| Black | 1.8 | 2.5 | 3.7 | |
| Asian | 2.7 | 2.8 | 1.8 | |
| Hispanic ethnicity | 3.8 | 4.8 | 4.1 | |
| Region of residence | ||||
| Northeast | 24.2 | 21.6 | 21.2 | |
| Midwest | 27.1 | 29.0 | 28.2 | |
| West | 22.6 | 16.4 | 23.7 | |
| South | 24.3 | 25.3 | 23.6 | |
| Smoking status | ||||
| Never smoker | 78.6 | 73.7 | 77.9 | |
| Former smoker | 4.2 | 6.3 | 5.4 | |
| Current smoker | 17.1 | 20.1 | 16.8 | |
| Married | 76.0 | 75.5 | 75.2 | |
| Nulligravidity | 61.0 | 49.1 | 58.4 | |
| Nulliparity | 73.1 | 63.1 | 71.7 | |
| Highest nursing degree | ||||
| PhD | 1.6 | 0.9 | 2.4 | |
| MSc | 24.9 | 26.5 | 19.6 | |
| RN or BSN | 71.1 | 69.0 | 73.6 | |
| LPN | 1.4 | 2.6 | 3.1 | |
| Associate’s degree | 0.7 | 0.4 | 1.1 | |
| Current student | 0.4 | 0.7 | 0.2 | |
| Typical work schedule | ||||
| Days only | 57.6 | 67.6 | 51.8 | |
| Evenings only | 4.8 | 7.1 | 5.8 | |
| Nights only | 17.8 | 9.1 | 19.9 | |
| Rotating with nights | 15.6 | 12.3 | 18.2 | |
| Rotating no nights | 4.1 | 4.0 | 4.3 | |
| Nursing work (hours/week) | ||||
| 1–20 | 7.7 | 13.1 | 5.4 | |
| 21–40 | 67.0 | 54.3 | 65.8 | |
| > 40 | 25.3 | 32.6 | 28.9 | |
| Moving or lifting a heavy load (times/day) | ||||
| 0 | 31.1 | 52.1 | 18.2 | |
| 1–5 | 40.9 | 28.8 | 46.1 | |
| 6–15 | 21.8 | 14.9 | 25.6 | |
| >15 | 6.2 | 4.2 | 10.1 | |
| Occupational exposures | ||||
| Antineoplastics | 9.3 | 7.7 | 16.7 | |
| Radiation | 2.5 | 4.6 | 11.0 | |
| Aerosol drugs | 0.6 | 0.2 | 2.7 | |
| Anesthetic gas | 7.8 | 10.8 | 15.2 | |
| Menstrual cycle length (days) | ||||
| ≤ 25 | 11.7 | 10.6 | 8.1 | |
| 26–31 | 86.1 | 87.8 | 90.8 | |
| 32–50 | 1.8 | 1.7 | 1.1 | |
| >50 | 0.4 | 0.0 | 0.0 | |
| Regular menstrual cycles | 79.6 | 80.9 | 77.0 | |
The median (25th, 75th percentile) current duration of pregnancy attempt in our cohort was 3 (1,11) months and the estimated proportions of women not pregnant after 12 and 24 months were 16% and 5%, respectively. Female nurses reporting ever use of HLD had an 18% (95% CI 5–31%) increase in the median duration of pregnancy attempt compared to women who never used HLD after multivariate adjustment for demographic, lifestyle, and occupational factors (table 2). This increase was observed among both women reporting exposure to HLD prior to baseline (TR=1.26, 95% CI 1.08–1.47) and current exposure to HLD at baseline (TR=1.12, 95% CI 0.98–1.28). Greater duration of HLD use was not linearly related to decreased fecundity as both women with short (<1 year) and long (≥6 years) durations of exposure had similar decreases in fecundity (TR=1.26 and 1.20, respectively). Women exposed to HLD at baseline for <1 hour per week had statistically significant longer median durations of pregnancy attempt compared to women who were never exposed (TR=1.16, 95% CI 1.00–1.35) while women exposed to HLD at baseline for ≥1 hour per week did not have any statistically significant differences in fecundity compared to women never exposed (TR=1.01, 95% CI 0.79–1.29). Exposure at baseline to any of the specific types of HLD was not associated with lower fecundity; however women exposed to an unknown type of HLD at baseline had a 27% (95% CI 5–54%) longer median duration of pregnancy attempt compared to women who were never exposed.
Table 2
Association between high-level disinfectants (HLD) use and fecundity among women attempting pregnancy in Nurses’ Health Study 3 (N=1739). [95% CI=confidence interval; REF=reference; TR=time ratio].
| N | % | Duration in months | Unadjusted | Adjusted a | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Median | Range | TR | 95% CI | TR | 95% CI | |||
| Ever used HLD? | ||||||||
| Never | 1202 | 69.1 | 3 | 1–8 | 1.0 | REF | 1.0 | REF |
| Yes | 537 | 30.9 | 4 | 1–12 | 1.26 | 1.12–1.41 | 1.18 | 1.05–1.31 |
| Ever used HLD? | ||||||||
| Never | 1202 | 69.1 | 3 | 1–8 | 1.0 | REF | 1.0 | REF |
| Yes, prior to baseline | 215 | 12.4 | 5 | 2–12 | 1.45 | 1.23–1.70 | 1.26 | 1.08–1.47 |
| Yes, at baseline | 322 | 18.5 | 3 | 1–11 | 1.15 | 1.00–1.32 | 1.12 | 0.98–1.28 |
| Duration of HLD use (years) | ||||||||
| 0 | 1202 | 69.1 | 3 | 1–8 | 1.0 | REF | 1.0 | REF |
| <1 | 103 | 5.9 | 4 | 1–12 | 1.32 | 1.05–1.65 | 1.26 | 1.02–1.57 |
| 1–5 | 291 | 16.7 | 3 | 1–11 | 1.13 | 0.97–1.30 | 1.14 | 0.99–1.31 |
| ≥6 | 143 | 8.2 | 5 | 1–24 | 1.52 | 1.26–1.85 | 1.20 | 0.99–1.45 |
| Frequency of HLD use in past month | ||||||||
| Never | 1202 | 69.1 | 3 | 1–8 | 1.0 | REF | 1.0 | REF |
| HLD use prior to baseline | 215 | 12.4 | 5 | 2–12 | 1.45 | 1.23–1.70 | 1.26 | 1.08–1.47 |
| <1 hour per week at baseline | 241 | 13.9 | 4 | 1–11 | 1.15 | 0.98–1.34 | 1.16 | 1.00–1.35 |
| ≥1 hour per week at baseline | 81 | 4.7 | 3 | 1–12 | 1.14 | 0.89–1.47 | 1.01 | 0.79–1.29 |
| Type of HLD used at baseline | ||||||||
| Never | 1202 | 69.1 | 3 | 1–8 | 1.0 | REF | 1.0 | REF |
| HLD use prior to baseline | 215 | 12.4 | 5 | 2–12 | 1.45 | 1.23–1.70 | 1.26 | 1.08–1.47 |
| Baseline HLD use - type unknown | 140 | 8.1 | 4 | 1–11 | 1.16 | 0.96–1.42 | 1.27 | 1.05–1.54 |
| Baseline HLD use - type known | 182 | 10.5 | 3 | 1–12 | 1.13 | 0.95–1.35 | 1.02 | 0.86–1.21 |
| Glutaraldehyde | 130 | 7.5 | 3 | 1–12 | 1.15 | 0.94–1.41 | 1.03 | 0.85–1.25 |
| Ortho-phthalaldehyde | 23 | 1.3 | 3 | 1–10 | 0.98 | 0.62–1.56 | 0.90 | 0.58–1.40 |
| Peracetic acid | 17 | 1.0 | 3 | 1–10 | 0.93 | 0.54–1.59 | 0.82 | 0.49–1.37 |
| Hydrogen peroxide | 55 | 3.2 | 3 | 1–11 | 0.97 | 0.72–1.32 | 0.85 | 0.64–1.14 |
| Number of protective equipment always/sometimes used | ||||||||
| No HLD use | 1202 | 69.1 | 3 | 1–8 | 1.0 | REF | 1.0 | REF |
| HLD use prior to baseline | 215 | 12.4 | 5 | 2–12 | 1.45 | 1.23–1.70 | 1.26 | 1.08–1.47 |
| Baseline HLD use - no protection | 94 | 5.4 | 3 | 1–11 | 1.12 | 0.89–1.42 | 1.18 | 0.93–1.49 |
| Baseline HLD use - some protection | 228 | 13.1 | 3 | 2–11 | 1.16 | 0.99–1.36 | 1.10 | 0.95–1.28 |
| 1 type | 149 | 8.6 | 3 | 2–11 | 1.16 | 0.95–1.40 | 1.16 | 0.97–1.39 |
| ≥2 types | 79 | 4.5 | 4 | 1–12 | 1.16 | 0.90–1.50 | 1.00 | 0.78–1.28 |
Of the nurses in our study who were exposed to HLD at baseline, 29% reported never using any type of PE, 46% reported using only one type of PE (98% of the time this was protective gloves), and 25% reported using ≥2 types of PE (supplementary table S1, www.sjweh.fi/index.php?page=data-repository). Use of ≥2 types of PE when exposed to HLD was more common among women who were older and heavier, had more frequent exposure to HLD, knew the type of HLD, were exposed to antineoplastics, and were not exposed to radiation. Among nurses exposed to HLD at baseline, use of ≥1 PE attenuated fecundity impairments (table 2). Specifically, women using 0, 1, and ≥2 types of PE had 18% (95% CI -7–49%), 16% (95% -3–39%), and 0% (95% -22–28%) longer median durations of pregnancy attempt compared to women who were never exposed to HLD.
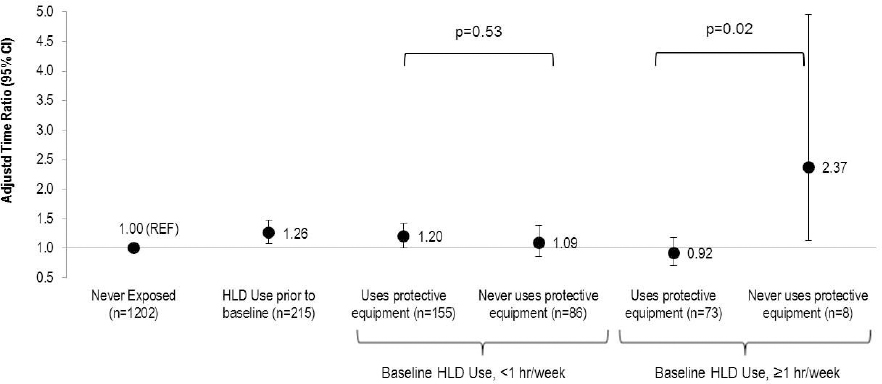
Factoring in the use of PE also helped explain the seemingly paradoxical finding that women with more frequent exposure to HLD at baseline had less of a fecundity impairment than women with less frequent HLD exposure, as 90% of nurses who used HLD ≥1 hour/week used PE compared to only 62% of nurses who used HLD <1 hour/week (figure 1). Among nurses exposed to HLD at baseline for ≥1 hour/week, those who never used PE (N=8) had more than double the duration of pregnancy attempt (TR=2.37, 95% CI 1.13–4.96) while those who used ≥1 PE had no statistically significant differences in duration of pregnancy attempt (TR=0.92, 95% CI 0.71–1.19) compared to nurses never exposed to HLD (P-value for difference=0.02). There was no statistically significant difference in the effect of exposure to HLD at baseline by use of PE among women using HLD for <1 hour per week. Similarly, women exposed to HLD at baseline who knew the type of HLD were more likely to use PE (92% for glutaraldehyde; 100% for ortho-phthalaldehyde; 88% for peracetic acid; 96% for hydrogen peroxide) compared to women who did not know the type of HLD (40%). While PE use attenuated the associations between HLD use and reduced fecundity among women with known type of HLD use, there was little difference in the effect estimates among women where the type of HLD was unknown (supplementary figure S2, www.sjweh.fi/index.php?page=data-repository).
Figure 1
Association between frequency of baseline high-level disinfectant (HLD) use and fecundity by use of personal protective equipment among women attempting pregnancy in Nurses’ Health Study 3 (N=1739). Accelerated failure time models with log normal distribution [adjusted for age, race, body mass index, smoking status, marital status, frequency of nursing work, frequency of lifting/moving a heavy load, and current exposure to radiation, antineoplastics, anesthesia gas, and aerosol drugs] were used to estimate the time ratios (TR) and 95% confidence intervals.
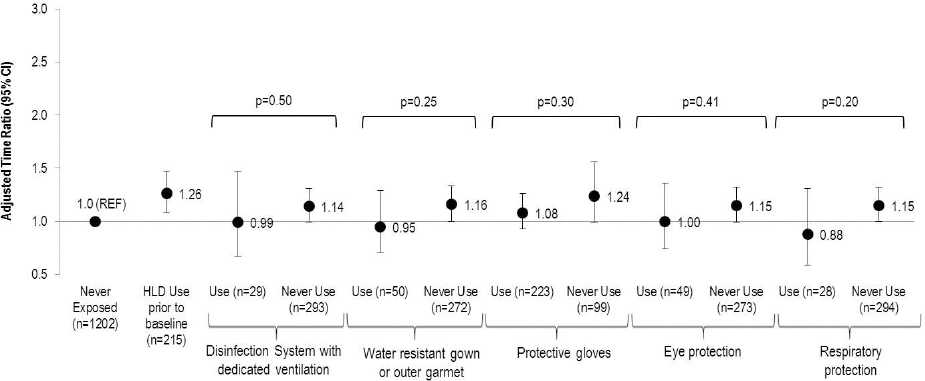
The use of PE among women exposed to HLD at baseline varied greatly by type: 69% for protective gloves, 16% for water resistant gowns or outer garments, 15% for eye protection, 9% for respiratory protection and 9% for disinfection systems with dedicated ventilation (figure 2). Use of each individual PE attenuated the association between HLD exposure and reduced fecundity; however none of the differences by type of PE were statistically significant.
Figure 2
Association between type of protective equipment use among those exposed at baseline to high-level disinfectants (HLD) and fecundity among women attempting pregnancy in Nurses’ Health Study 3 (N=1739). Accelerated failure time models with log normal distribution [adjusted for age, race, body mass index, smoking status, marital status, frequency of nursing work, frequency of lifting/moving a heavy load, and current exposure to radiation, antineoplastics, anesthesia gas, and aerosol drugs] were used to estimate the time ratios (TR) and 95% confidence intervals.
The association between past and current HLD use at baseline and decreased fecundity was consistent when the analysis was restricted to women reporting a pregnancy attempt within two years of exposure assessment, when the outcome distribution was changed to generalized gamma, and when we further adjusted the multivariate models for gravidity (supplementary table S2, www.sjweh.fi/index.php?page=data-repository). As expected, the TR for the effects of past and current HLD use at baseline on fecundity were slightly lower in magnitude (although still elevated) after we truncated current durations of pregnancy attempt >12 months to 12 months. There was no evidence of effect modification of the association between HLD use and fecundity across strata of age, BMI, smoking status, or gravidity.
Discussion
In this ongoing cohort of female nurses planning pregnancy, occupational use of HLD was associated with reduced fecundity, but use of PE appeared to attenuate this relation. Nevertheless, due to the lack of previous human data and the relatively modest effect sizes, these findings should be interpreted with caution. It is important that this question is addressed in other studies in order to determine and fully characterize its nature and magnitude. The use of PE among women exposed to HLD varied greatly by type (9–69%). Among the women who used PE, 98% wore protective gloves.
Our finding that exposure to HLD is associated with reduced fecundity is consistent with animal studies on exposure to ethylene oxide and formaldehyde, two sterilants that have been largely phased out within the healthcare setting. Rates exposed to ethylene oxide via inhalation prior to mating had reduced fertility (6) and a reduced number of corpora lutea, implantations, and live fetuses (9). Pregnant rats exposure to formaldehyde via inhalation had damaged blastomeres (28), a decrease in placenta and corpus luteum size, and increased fetal abnormalities (29). While none of the nurses in our study reported exposure at baseline to ethylene oxide or formaldehyde, it is possible the nurses reporting former exposure to HLD, who had a 26% reduction in fecundity compared to nurses who were never exposed, handled these specific chemical agents. Yet, since we lack information on the specific types of HLD used by these women prior to baseline, this is only speculation.
There have only been four animal studies and no epidemiologic studies on the potential reproductive toxicity of exposure to glutaraldehyde, the most commonly reported HLD in our study. The first study demonstrated that only very high doses (eg, 5 ml/kg/day) of potentiated acid glutaraldehyde increased the number of stunted and malformed fetuses (8). The second study in rats, however, using similar doses and administration route showed no significant differences in the number of implantations or post-implantation losses per litter (10). The third study demonstrated that rats exposed to glutaraldehyde by inhalation spent more time in estrus and diestrus and less time in metestrus and proestrus than controls (30). However, in the most recent study, rats exposed to glutaraldehyde through water found no effects on fertility, mating performance, pup viability, or litter size (11). To our knowledge, there have not been any studies on the reproductive toxicity of exposure to ortho-phthalaldehyde (OPA), peracetic acid, or hydrogen peroxide, HLD that were introduced more recently as safer alternatives to glutaraldehyde. In our study, we found no adverse effects of exposure when looking at each specific type of HLD (glutaraldehyde, ortho-phthalaldehyde (OPA), peracetic acid, or hydrogen peroxide); although >88% of women using these HLD were also using PE which could have reduced exposure and any potential reproductive toxicity.
While research linking past exposure to HLD and current fecundity is limited, there are some plausible mechanisms. Exposure to chemicals prior to conception may damage reproductive organs and/or germ cells that could affect reproduction and/or damage the genetic makeup of the woman’s gametes. For instance, exposure to formaldehyde in female rats resulted in decreased ovarian weights, increased levels of serum LH and FSH, endometrial hypoplasia, and lack of ovarian luteal tissue (31, 32). Numerous other studies have also shown that formaldehyde induces genotoxic and mutagenic effects on cells throughout the body (33). Taken together these results in animals suggest that pre-conception exposure to formaldehyde (and possibly other HLD) could result in lasting effects to fecundity; however, research further investigating this question in humans is needed.
Our finding that use of PE attenuated the reduced fecundity we observed with exposure to HLD is consistent with research showing that proper use of PE reduces exposure to these chemicals through inhalation and dermal absorption (34, 35). An alarming finding, however, was that 29% of nurses exposed to HLD at baseline did not use any type of PE. This is concerning as the Occupational Safety and Health Administration’s Hazard Communication Standard (29CFR1910.1200) requires employers to educate their employees on the hazards of these types of chemicals and proper PE use in addition to safe handling practices, emergency procedures, and how to use safety data sheets. In a study on precautionary practices of healthcare workers who disinfect medical devices, the most common reasons for not using PE when handling HLD was “exposure was minimal” or “not part of our protocol” (36). These respondents were also more likely to have never received training on the safe handling of HLD (36). Our finding that women with less frequent exposure to HLD were less likely to use PE is consistent with these previous findings and suggests that employers should further emphasize consistent and effective use of PE, even when exposure is infrequent.
We found none of the specific HLD were associated with fecundity impairments. While this seemed to be explained by the high use of PE among nurses who knew the HLD being used, exposure to HLD among nurses who did not know the HLD used was associated with longer current duration of pregnancy attempt regardless of PE use. We have several potential explanations for these findings. First, nurses who were unaware of the specific type of HLD were much less likely to report use of HLD for ≥1 hour/week compared to users of a known type (14% versus 34%). Given this infrequent use, it is plausible that these women were also less likely to have been trained in the safe handling of HLD and proper use of PE. Thus, even among nurses who were unaware of the specific type of HLD and reported always or sometimes using PE, it may not have been used as effectively to prevent exposure. Second, it could be that these nurses misinterpreted the question on disinfection use and were in fact reporting exposure to other types of low-level disinfectants rather than HLD. Given the reports relating quaternary ammonium disinfectants and subfertility in animals (37, 38), it is possible that these low-level disinfectants could also be associated with fecundity impairments among humans. Finally, it is possible that residual or unmeasured confounding by other occupational or lifestyle factors is explaining this association.
In lieu of our statistically significant findings, it is important to consider two important potential biases, since our study consisted entirely of working women who were planning a pregnancy. If past or current use of HLD at baseline was associated with unplanned pregnancies and if these unplanned pregnancies also had longer or shorter waiting times to pregnancy this could have biased results in either direction (39). To address this possibility, we looked at pregnancy planning among women enrolled in our Maternal Health Study, a sub-study of pregnant participants within the NHS3 cohort. Women with planned (76%) and unplanned (24%) pregnancies reported similar exposure to HLD and use of PE, indicating that any planning bias is likely minimal for these exposures. The second bias worth considering is termed the “infertile worker effect” (40). Women who have not had a successful pregnancy are more likely to remain in the workforce and may have more opportunity for occupational exposure than women who have had successful pregnancies, who may leave the workforce to care for children (41). While this type of potential bias is harder to address directly, our results remained statistically significant after adjustment for many of the socioeconomic variables associated with employment status, and there was no significant difference in effect when we restricted analyses to nulliparous women. Thus, it seems unlikely that the infertile worker effect is strongly biasing our findings.
It is possible the questions about HLD use on the questionnaire lacked specificity, which may have contributed to exposure misclassification. Moreover while we had information on frequency of HLD and PE use, total exposure to HLD is related to numerous physical workplace factors that we did not collect information on, such as contact duration, HLD concentration in the sterilizing solution, size of the room, and general room ventilation or airflow (42). We also assessed exposure only at baseline and assumed this exposure was constant for the duration of the woman’s pregnancy attempt. If there were changes in the use of HLD in response to having experienced longer pregnancy attempts, this could have resulted in exposure misclassification. Fortunately, exposure was assessed ≥6 months prior to pregnancy duration assessment, thus it is unlikely that this exposure misclassification was differential with respect to duration of pregnancy attempt. Second, women who reported currently attempting pregnancy on the baseline questionnaire did not differ in self-report of exposure to HLD, frequency of HLD use, or frequency of PE use compared to women who were not currently planning pregnancies suggesting that the women in our cohort did not change their occupational exposures or behaviors while attempting pregnancy. Since this was a secondary analysis of existing data, we lacked information on other possibly important confounders, such as frequency of sexual intercourse or characteristics of the male partner. Co-exposure to other work-related stresses, including hazardous substances and physical hazards, were also not measured (other than co-exposure to radiation, antineoplastic, anesthesia gas, and aerosol drugs). Thus, we cannot completely rule out that the observed associations were due to residual or unmeasured confounding, as mentioned previously. Given the relatively modest effect sizes we observed, we also cannot rule out the possibility that these are chance findings. We also did not have information on current use of infertility treatment. If use of infertility treatment shortens or lengthens a woman’s duration of pregnancy attempt and likelihood of treatment is associated with HLD exposure, then our results could be biased in either direction. Of note, we tried to minimize the effect of this by assigning all women with a current duration of pregnancy attempt >3 years to 3 years and in sensitivity analyses we changed this cut-off to 1 year. In all of these analyses, results remained similar.
Our study had several strengths. Since the use of HLD in healthcare is necessary for the disinfection of medical equipment and unlikely to be completely eliminated, collecting information on use of PE during HLD exposure allowed us to address the more clinically relevant question of whether the association between HLD use and fecundity was modified by PE. Second, by using a current duration approach, as compared to more traditional time to pregnancy approaches, we were able to include both women with high fertility (who are excluded from many prospective cohorts) and those who are involuntarily infertile (who are excluded from retrospective pregnancy cohorts). Finally, due to the homogenous nature of this cohort (eg, all nurses with some level of health-related education), many socio-economic factors were controlled for in the design of this cohort.
In conclusion, occupational use of HLD was associated with reduced fecundity among nurses. Use of PE, particularly the use of ≥1 PE, appeared to attenuate the adverse effects of exposure to HLD on fecundity, suggesting that equipment designed to reduce dermal contact and inhalation may be effective in minimizing the health effects of these chemicals. Given the observational nature of this cohort and the relatively modest effect sizes we observed, the possibility that these associations are chance findings cannot be ruled out. Future research, in animal and human models, is needed to better understand whether the association between HLD currently being used in healthcare facilities and fecundity exists and if so, the biological mechanisms underlying these associations.



