A number of studies have identified that exposures to solvents (1–4) and metals (5) can influence respiratory symptoms. However, only a few have investigated these exposures in relation to fixed airflow obstruction (AO) that is used to define chronic obstructive pulmonary disease (COPD) in epidemiological literature (6, 7).
Solvents are routinely used in industry and are present in products such as fuels, paints, printing, degreasing metal parts, and cleaning products (8, 9). Solvents can be further divided into “aromatic solvents” such as benzene and “chlorinated solvents” including trichloroethylene, and tetrachloroethylene (4, 9). On inhalation, solvents slowly damage the airways causing mucus hypersecretion and may subsequently lead to airflow obstruction (10). Population-based studies have suggested a link between occupational exposure to solvents and chronic nonspecific lung disease (1–3, 11) and reduced lung function using pre-bronchodilator (BD) spirometry (4, 11). However, their findings are limited by their reliance on self-reported exposure and the use of physician’s diagnosis to define chronic nonspecific lung disease. The Dutch Life Lines Cohort Study found an association between chlorinated solvents and chronic mucus hypersecretion in participants without AO (7), but no association between chlorinated solvents and lower lung function and AO (6). Limitations of these studies included the use of pre-BD to define AO when the recommended criterion is post-BD (12) and the use of current job rather than a complete job history which allows assessment of cumulative exposure (13).
Metals are also commonly used in a variety of industrial processes (14) and, via the manipulation of metal products, metal form aerosols can be inhaled (15). Population-based studies have suggested an association between occupational exposure to metals and chronic nonspecific lung disease (1, 7). Only two population-based studies have assessed the relationship between metals exposure and AO defined using lung function measurements, and neither found a significant association (4, 7). However, these studies were again limited by the use of pre-BD spirometry to define AO.
We investigated the associations between exposures to solvents and metals and fixed AO defined by post-BD spirometry and used a general population-based job exposure matrix (JEM) to calculate ever-exposure and cumulative exposure-unit (EU) years.
Methods
Data collection
Our study included participants from the most recent laboratory phase of the Tasmanian Longitudinal Health Study (TAHS) for which the methodology has been reported previously (16). In brief, the TAHS began in 1968 when 8583 Tasmanian children born in 1961 and attending school in Tasmania were enrolled by their parents who completed a respiratory health questionnaire for the child, who then underwent clinical examinations and lung function measurements. Data for the current analysis were collected at the follow-up survey started in 2002. The follow-up began with tracing 7562 (88.1%) of the original 1968 cohort to an address and achieving a response of 5729 (78.4%) to a postal survey (16). A subgroup of these respondents, selected on the basis of their participation in previous follow-ups, and an enriched sample with a history of asthma and chronic bronchitis were invited to participate in a detailed laboratory study between 2005 and 2008. Of the 2387 individuals invited, 1397 (58.6%) attended the laboratory and completed lung-function testing, 354 (14.8%) completed a telephone questionnaire including lifetime work history calendar but did not attended the laboratory to complete lung-function testing and remaining 636 (26.7%) withdrew. At the laboratory visit, participants completed a detailed interviewer-administered respiratory health questionnaire, completed a lifetime work history calendar, performed pre- and post-BD lung-function tests, skin-prick testing, lung volumes, and diffusing capacity test.
Lung-function testing
Pre and post-BD lung function were measured using the Easyone Pro® ultrasonic spirometer (ndd, Medizintechnik, AG, Switzerland). Participants were asked not to smoke for 4–6 hours before testing. Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were measured according to the American Thoracic Society (ATS) and European Respiratory Society (ERS) joint statement (17) and the highest value for FEV1 and FVC was recorded from three acceptable and repeatable tests. Spirometry was repeated 10–15 minutes after short-acting bronchodilator (200 μg salbutamol) administered via a spacer.
Single breath diffusing capacity of the lung for carbon monoxide (DLCO) was measured according to international guidelines and adjusted to a standard haemoglobin concentration of 14.6 g/dL in men and 13.4 g/dL in women and corrected for the presence of carboxyhemoglobin (18). The average of two acceptable tests that agreed to within 10% was recorded. Predicted values of DLCO were calculated using the prediction equations of Thompson and colleagues (19).
Lung-function definitions
Fixed AO was defined in two ways: first, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) fixed cut-off criterion (FEV1/FVC<0.7) (20) and second using FEV1/FVC<lower limit of normal (LLN) as based on the Global Lung Initiative (GLI) 2012 reference equations (21).
Fixed AO plus low DLCO was defined by FEV1/FVC<0.7 and DLCO <80% of predicted value (22).
Occupational exposures
Occupational exposure were classified based on a full job history that was completed during the laboratory visit or telephone interview. Participants were asked to list all the jobs held over their lifetime. We collected data including job title, industry type, company name, the year when job started and ended from 1599 participants. In this analysis, we included 1335 participants those have both work history calendar and completed lung-function testing at the 2002–2008 follow-up. The jobs reported in the calendars were coded according to the International Standard Classification of Occupations (ISCO-88) four- digit classification (23). These codes were then used to assign occupational exposures to solvents (three categories: aromatic solvents, chlorinated solvents, and other solvents) and metals using the ALOHA plus JEM (6, 24). The JEM classified subjects based on job code into no, low and high exposure.
Ever-exposure
We combined low and high exposure categories because the small number of participants in the high-exposed group limited the statistical analysis and assigned people as “ever exposure”, if they had occupational exposure for any job over their working life (25).
Cumulative EU years
We estimated this by the number of years worked in a given job multiplied by the exposure intensity for the given job and summed for each individual and each exposure across all jobs performed. To allow for the combination of low and high exposure categories, years of exposure was weighted by four for high exposure and one by low exposure to calculate cumulative EU years (24).
Statistical methods
All the statistical analyses were performed using Stata 13.1 (Stata Corporation, College Station, TX, USA) statistical package. Multinomial logistic and linear regression models were used to analyzed the associations between occupational exposures and fixed AO (categorical) and lung-function measures (continuous). The lung function variables were normally distributed. The final multinomial model was adjusted for sex, smoking, pack-years, asthma in childhood and adulthood, and socioeconomic status in childhood and adulthood (see online supplementary material for details, www.sjweh.fi/index.php?page=data-repository). Sampling weights were derived by calculating the inverse probability of selection for the enriched sample of the 2002 to 2008 follow-up.
To investigate potential effect measure modification by sex, smoking, and current asthma, additive log risk model were compared with multiplicative risk ratio model using likelihood ratio test and Bayesian information criterion. We did not identify any effect measure modification between occupational exposure and smoking or current asthma status (results not shown). However, we did observe some effect measure modification with sex so results for the association between cumulative EU years and fixed AO are stratified by sex. We excluded the participants with missing or incomplete years started or ended (N=80) from the cumulative EU years analysis. All complete case analysis were additionally compared with multiple imputation for dealing with missing data (data not shown). A cut-off of P<0.05 was considered as statistically significant.
We examined the correlation between solvents and metals exposure with other exposures from the ALOHA plus JEM; vapors, gases, dust, and fumes (VGDF) and all pesticides exposures and did not see a strong correlation between any of these variables (data not shown). Additionally, in our final adjusted regression model, we included VGDF, and pesticides exposures as an additional confounder to observe if they changed the effect estimates. As the addition of these variables into the models did not change in the effect estimates by 10% or more (data not shown), we did not adjust our final models for these factors.
Results
The characteristics of the study population are presented in table 1. The mean age of participants was 44.8 years, and more than half (51.6%) were men. Men had a higher median smoking pack-years than women (table 1) and 25% men were current smokers, compared with 18% of women. A comparison of non-responders to different parts of the 2002 to 2008 follow-up study is shown in supplementary table S1. Briefly selected characteristics of the participants who did and did not attend laboratory were similar. Men were more likely to have had ever occupational exposure to solvents and metals than women (supplementary table S2).
Table 1
Study characteristics stratified by sex. [SD=standard deviation; IQR=interquartile range; GOLD=global initiative for chronic obstructive lung disease; LLN=lower limit of normal; DLCOdiffusing capacity of the lung for carbon monoxide.]
Ever-exposure
Ever-exposure to chlorinated solvents was associated with an increased risk of fixed AO [relative risk (RR) 1.45, 95% CI 0.91–2.34] and fixed AO plus low DLCO (RR 2.19, 95% CI 0.98–4.91) with both approachings but not quite reaching statistical significance (table 2). Ever-exposure to metals was also significantly associated with an increased risk of fixed AO (RR 1.71, 95% CI 1.03–2.85) but the evidence for its association with of fixed AO plus low DLCO was modest (RR 2.04, 95% CI 0.83–5.04).
Table 2
Associations between ever-exposures, fixed airflow obstruction (AO) and fixed AO plus low diffusing capacity of the lung for carbon monoxide (DLCO). [GOLD=global initiative for chronic obstructive lung disease; LLN=lower limit of normal; RR=relative risk.]
| Exposure | Fixed AO-GOLD | Fixed AO-LLN | Fixed AO plus low DLCO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| No (N=1222) | Yes (N=113) | RR a | 95% CI | No (N=1223) | Yes (N=112) | RR | 95% CI | No (N=1164) | Yes (N=35) | RR | 95% CI | |
| Aromatic solvents | ||||||||||||
| Not exposed | 873 | 70 | Ref. | 873 | 70 | Ref. | 827 | 24 | Ref. | |||
| Exposed | 349 | 43 | 1.21 | 0.76–1.93 | 350 | 42 | 1.18 | 0.74–1.88 | 338 | 11 | 0.91 | 0.39–2.12 |
| Chlorinated solvents | ||||||||||||
| Not exposed | 964 | 78 | Ref. | 964 | 78 | Ref. | 914 | 23 | Ref. | |||
| Exposed | 258 | 35 | 1.45 | 0.91–2.34 | 259 | 34 | 1.40 | 0.87–2.28 | 251 | 12 | 2.19 | 0.98–4.91 |
| Other solvents | ||||||||||||
| Not exposed | 749 | 58 | Ref. | 749 | 58 | Ref. | 710 | 19 | Ref. | |||
| Exposed | 473 | 55 | 1.34 | 0.89–2.06 | 474 | 54 | 1.32 | 0.87–2.02 | 455 | 16 | 1.24 | 0.60–2.57 |
| Metals | ||||||||||||
| Not exposed | 948 | 72 | Ref. | 948 | 72 | Ref. | 898 | 22 | Ref. | |||
| Exposed | 274 | 41 | 1.71 | 1.03–2.85 | 275 | 40 | 1.67 | 1.00–2.78 | 267 | 13 | 2.04 | 0.83–5.04 |
We also investigated the association between ever-exposure to aromatic solvents, chlorinated solvents, other solvents, metals and lung function measures (FEV1, FVC, FEV1/FVC, and DLCO) (supplementary table S3). We found significant association with lower FEV1/FVC ratio for those with ever-exposure to metals (z-score FEV1/FVC ratio; -0.17, 95%CI -0.33– -0.02), but we found no other significant associations (supplementary table S4).
We found the similar association between ever exposed to metals and pre-BD FEV1/FVC<0.7 (RR 1.47, 95% CI 1.00–2.21) and FEV1/FVC<LLN (RR 1.57, 95% CI 1.04–2.39) (supplementary table S5). Additionally, we found a significant association with other solvents (RR 1.39, 95% CI 1.00–1.93) and pre-BD airflow obstruction.
Effect measure modification and cumulative EU-years
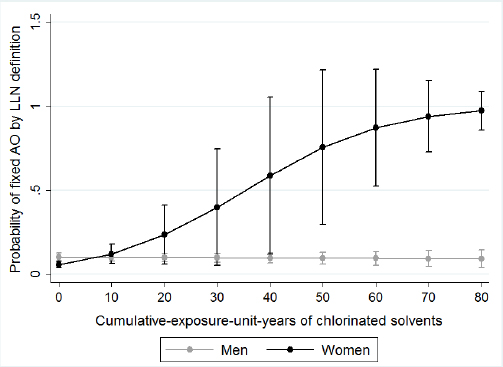
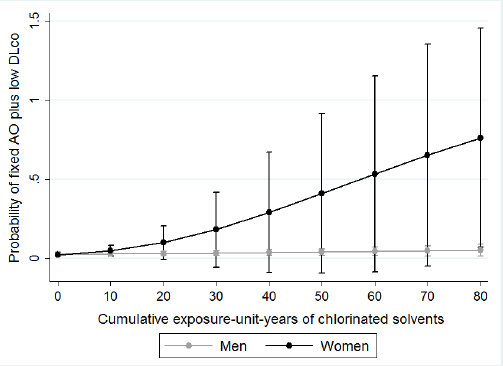
We examined effect measure modification by sex, asthma status, and smoking status. We presented the associations between cumulative EU years and fixed AO and fixed AO plus low DLCO stratified by sex in table 3. We found a significant effect measure modification between sex and cumulative EU years to chlorinated solvents on the risk of fixed AO-GOLD and fixed AO-LLN. We found women have less cumulative EU years to chlorinated solvents [mean 20.9, standard deviation (SD) 13.4] compared to men (mean 28.6, SD 36.9), but women have modified the association between cumulative EU years to chlorinated solvents and fixed AO-GOLD and fixed AO-LLN (RR 1.08, 95% CI 1.03–1.15) than men. Figures 1 and 2 displays the predicted probabilities of fixed AO that were computed using the estimates from the multivariate regression models. In this model, women with increasing chlorinated solvent EU years had a significantly increased risk of fixed AO. However, for men, we did not observe any such relationship. We observed a similar effect measure modification by sex for fixed AO plus low DLCO (P-value for effect measure modification=0.02, figure 3). We did not observe that sex modifed the effect of other exposures or any effect modification by sex, smoking or asthma status for exposures to either solvents or metals and fixed AO. Sex did not modify any associations between any of the solvents or metals and AO when defined using pre-BD lung function (data not shown).
Table 3
The association between cumulative exposure-unit (EU) years and fixed airflow obstruction (AO) and fixed AO plus low diffusing capacity of the lung for carbon monoxide (DLCO). stratified by sex and adjusted for smoking, pack-years, childhood and adulthood socioeconomic status, and childhood and adulthood asthma. [GOLD=global initiative for chronic obstructive lung disease; LLN=lower limit of normal; RR=relative risk; SD=standard deviation.]
| Exposure | Women | Men | P-value a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| N | Cumulative EU years | RR | 95% CI | N | Cumulative EU years | RR | 95% CI | ||||
|
|
|
||||||||||
| Mean | SD | Mean | SD | ||||||||
| Aromatic solvents | |||||||||||
| Fixed-AO-GOLD | |||||||||||
| No | 559 | 7.1 | 6.9 | Ref | 589 | 17.4 | 16.6 | Ref | |||
| Yes | 39 | 38.3 | 57.1 | 1.02 | 0.98–1.07 | 68 | 13.4 | 11.2 | 0.99 | 0.97–1.01 | 0.17 |
| Fixed-AO-LLN | |||||||||||
| No | 559 | 7.1 | 6.9 | Ref | 590 | 17.5 | 16.6 | Ref | |||
| Yes | 39 | 38.3 | 57.0 | 1.03 | 0.99–1.07 | 67 | 12.7 | 10.5 | 0.99 | 0.97–1.01 | 0.14 |
| Fixed AO + low DLCO | |||||||||||
| No | 514 | 6.6 | 6.1 | Ref | 579 | 17.1 | 16.5 | Ref | |||
| Yes | 14 | 38.3 | 57.0 | 1.05 | 0.98–1.14 | 18 | 17.5 | 10.9 | 0.99 | 0.95–1.03 | 0.05 |
| Chlorinated solvents | |||||||||||
| Fixed-AO-GOLD | |||||||||||
| No | 559 | 8.1 | 6.7 | Ref | 589 | 40.9 | 40.4 | Ref | |||
| Yes | 39 | 20.9 | 13.4 | 1.08 | 1.03–1.15 | 68 | 28.6 | 36.9 | 0.99 | 0.98–1.00 | 0.006 |
| Fixed-AO-LLN | |||||||||||
| No | 559 | 8.1 | 6.7 | Ref | 590 | 40.7 | 40.4 | Ref | |||
| Yes | 39 | 20.9 | 13.4 | 1.08 | 1.03–1.15 | 67 | 29.3 | 37.4 | 0.99 | 0.98–1.00 | 0.006 |
| Fixed AO + low DLCO | |||||||||||
| No | 514 | 8.8 | 8.2 | Ref | 579 | 38.4 | 39.5 | Ref | |||
| Yes | 14 | 23.7 | 5.3 | 1.08 | 1.01–1.17 | 18 | 47.3 | 47.7 | 1.00 | 0.99–1.02 | 0.02 |
| Other solvents | |||||||||||
| Fixed-AO-GOLD | |||||||||||
| No | 559 | 16.5 | 17.2 | Ref | 589 | 17.8 | 18.0 | Ref | |||
| Yes | 39 | 19.1 | 19.9 | 1.01 | 0.99–1.03 | 68 | 12.5 | 10.0 | 0.99 | 0.97–1.01 | 0.17 |
| Fixed-AO-LLN | |||||||||||
| No | 559 | 16.6 | 17.2 | Ref | 590 | 18.1 | 18.4 | Ref | |||
| Yes | 39 | 18.9 | 19.2 | 1.01 | 0.99–1.03 | 67 | 11.6 | 9.2 | 0.98 | 0.96–1.00 | 0.12 |
| Fixed AO + low DLCO | |||||||||||
| No | 514 | 17.0 | 17.6 | Ref | 579 | 17.5 | 17.9 | Ref | |||
| Yes | 14 | 21.0 | 27.3 | 1.02 | 0.99–1.04 | 18 | 17.6 | 10.1 | 0.99 | 0.96–1.02 | 0.27 |
| Metals | |||||||||||
| Fixed-AO-GOLD | |||||||||||
| No | 559 | 9.3 | 11.5 | Ref | 589 | 34.4 | 38.3 | Ref | |||
| Yes | 39 | 27.0 | 22.5 | 1.05 | 0.98–1.12 | 68 | 24.1 | 33.4 | 0.99 | 0.99–1.00 | 0.17 |
| Fixed-AO-LLN | |||||||||||
| No | 559 | 9.3 | 11.5 | Ref | 590 | 34.3 | 38.3 | Ref | |||
| Yes | 39 | 27.0 | 22.5 | 1.05 | 0.98–1.13 | 67 | 24.4 | 33.8 | 0.99 | 0.99–1.00 | 0.17 |
| Fixed AO + low DLCO | |||||||||||
| No | 514 | 12.1 | 14.3 | Ref | 579 | 32.1 | 37.1 | Ref | |||
| Yes | 14 | 20.5 | 27.6 | 0.87 | 0.39–1.94 | 18 | 38.7 | 46.8 | 1.00 | 0.99–1.02 | 0.58 |
Figure 1
The associations between years of chlorinated solvents exposure and probability of fixed AO-GOLD, stratified by sex. Circles represent the probability of the outcome (fixed AO), and the bars represent the 95% confidence interval of that probability.
Discussion
Our study is the first to assess occupational exposures to solvents and metals examining both ever-exposure and cumulative EU years in this cohort. The major new finding is that increasing cumulative EU years to chlorinated solvents is significantly associated with increased risks of fixed AO for women but not men although mean cumulative EU years to chlorinated solvents was less among women. The observed associations with fixed AO were restricted to the cumulative EU years and not ever being exposed to aromatic, chlorinated, and other solvents. However, we did find that ever-exposure to metals was associated with fixed AO and a lower FEV1/FVC ratio, but no association was observed with cumulative EU-years to metals.
To our knowledge, ours is the first study to show that cumulative EU years to chlorinated solvents is associated with fixed AO and fixed AO plus low DLCO in women. The only study that assessed the association between ever- (low or high) exposure to chlorinated solvents and AO was limited to pre-BD lung function measures, which do not allow for clarifying the fixed nature of the airflow obstruction (6). However, similar to the LifeLines Cohort Study, we did not find any association between chlorinated solvents and AO defined using pre-BD airflow obstruction (6) nor any an effect modification by sex.
The different job distribution between men and women in our study may be another reason for increased risk among women. In our study, women exposed to chlorinated solvents worked as dry cleaners, laundry pressers, sculptors, painters, decorators and commercial designers, chemist, hairdresser or beauticians and chemical laboratory science technicians, while men were mostly metal, rubber, plastic product assemblers, glass, ceramics painters, mechanical and electrical technicians, painters, and sheet metal workers. Exposure to specific types of chlorinated solvents has been associated with symptoms of cough and phlegm in female laundry employees and dry cleaners (26). One might speculate that the increased risk of airflow obstruction among women who had occupational exposure to chlorinated solvents in our study might be related to an increased number for women to work in jobs with exposure to chlorinated solvents such as in the dry-cleaning or painting or pharmaceuticals industries. However, we actually found the women in our study had a lower prevalence of exposure and had fewer years of cumulative exposure to chlorinated solvents than men had. This raises the possibilities that the observed association could be related to the different types of chlorinated solvents used in different jobs undertaken by men and women, or alternatively, a greater degree of susceptibility to chlorinated solvents in women.
A greater susceptibility for women to develop fixed AO from inhaling respiratory pollutants has been debated over past decades (27). Postulated mechanisms include more toxin being deposited per surface area of the lungs, impaired clearance of the toxin substance or a modified biological response to the inhaled toxin (27, 28). Evidence from studies of smoking has shown that women receive a greater dose of toxin for the same amount of inhaled smoke because of their smaller airway size (29). Toxins in the airways are metabolized via the cytochrome P450 pathway (27). The female sex hormone estrogen can influence this pathway by causing the up-regulation of the CYP1A1, CYP1A2, CYP2A6, CYP3A4, and CYP1B1 cytochromes (28, 30). The role of these enzymes in the toxin metabolism pathway leads to increased oxidation of inhalation leading to increased oxidative stress in the airways and greater risk of airflow obstruction in women (28). A recent in vivo animal study in mice suggested that female mice were more susceptible to airway remodeling with narrowed airway lumens and thickened airway walls compared to men, which they hypothesized was due to a greater susceptibility to oxidative stress due to the presence of the female sex hormone, estrogen (31). Studies among humans conflict with one study by Matheson et al (24) that found an increased risk of airflow obstruction among women exposed to biological dust but no increased risk among men. In contrast, some previous studies have assessed occupational exposure among men and women separately and have not observed any differences by sex (32, 33). A possible reason for this difference could that the latter two studies used pre-BD spirometry, and their study participants were younger and therefore less likely to have developed fixed AO. Importantly, none of these studies investigated chlorinated solvents exposure, and it may be that the chemical structure of chlorinated solvents increases the risk among women but not men. Overall, our findings add to the limited body of evidence that supports women as being more susceptible to the adverse lung function effect from specific occupational exposures.
Along with fixed AO, we also found an increased risk of fixed AO plus low DLCO with increasing cumulative EU years to chlorinated solvents. Coexistent low DLCO indicates the presence of emphysema as a diagnosis of emphysema requires either radiological evidence on computed tomography scan or a reduced gas transfer factor in the presence of chronic airflow obstruction (22). Others have also shown that some other occupational exposures have been associated with pulmonary emphysema at autopsy (34) or detected by computed tomography imaging (35). To our knowledge, no previous study has assessed the association between chlorinated solvents and emphysema. One previous study that used this definition of asymptomatic emphysema (airflow obstruction plus low DLCO) found women with biological dust exposure were at an increased risk of emphysema (24). However, as we combined the definition of fixed AO with low DLCO, there is a chance that the participants with fixed AO may have influenced or contributed in the combination of the two outcomes rather than gas transfer factor itself.
Ever-exposure to metals was associated with lower level of FEV1/FVC and a greater risk of fixed AO in our study, and this is consistent with previous studies that found an increased risk of respiratory symptoms (7) and lung function decline (5, 36, 37) with exposure to metals in welders. For steelworkers, cumulative respirable metal dust exposure may cause pulmonary surfactant impairment resulting in lung function decline and chronic respiratory diseases (5). Subjects exposed to metals in our study were mainly working in metal or steel industries such as motor vehicle mechanics and fitters, sheet metal worker, welders and flame cutters and agriculture or industrial machinery mechanics. Our observation of an association between ever-exposure to metals was similar to previous studies that found an association between any exposure (high or low) to metals with lung function and chronic lung disease (1, 4, 7). However, due to lack of lifetime work history calendar, previous studies were unable to assess cumulative exposure (1, 4, 7).
The major strengths of our study include the use of post-BD spirometry to define fixed AO, the availability of lifetime work histories for the participants’ entire working life, the use of a general population -based ALOHA plus JEM and adjustment for important potential confounding factors collected prospectively over time. The lifetime work histories allowed us to calculate ever and cumulative EU years and reduces the chance of recall bias as the participant is asked to recall their entire work history not just jobs they may recall as having high exposure to a possible causative agent. The main limitations of our study include possible sources of information bias, including recall and misclassification bias, and selection bias such as non-response bias. There is the possibility of recall or “exposure suspicion” bias caused by the participant’s selective recall of exposures. We have at least reduced the possibility of this type of bias using a JEM based on lifetime work history calendars that collected information on all jobs the participant has ever held irrespective of the possibility of exposure. JEM themselves may be affected by non-differential misclassification bias of the exposure due to the heterogeneity of exposure within a given job title or occupation, but, if present, such non-differential misclassification would be expected to underestimate the true effects of exposure. Furthermore, the interviewing staff and participants were blinded to the occupational exposure hypothesis reducing the possibility of information biases. The next possible type of bias in our study is selection or non-response bias or “healthy volunteer” bias, caused by the participants choosing to participate in the laboratory study. Participants who self-select to participate in a study have in general better health than those who do not. We cannot fully exclude this type of bias however we did adjust all analysis for factors found to differ between those who attended the laboratory and those who did not. Based on these limitations, we suggest that our results should be viewed as hypothesis-generating rather than proof that these exposures are causal and replication in another cohort is needed.
In conclusion, our study has shown that cumulative EU years to chlorinated solvents is associated with increased risk of post-BD fixed AO among women but not men. We have also shown that this association is not due to greater exposure of women and suggest that this may be due to their greater susceptibility. We also found that ever-exposure to metals was associated with fixed AO. Our study emphasizes the need to utilize post-BD spirometry to define fixed AO which allows adequate consideration of asthma, especially for a middle-aged population. Our findings highlight the need to reduce workplace exposure to solvents and metals by eliminating the source of exposure origin, improving adherence to use of recommended protective equipment, and workplace monitoring of exposure levels could be implemented. Preventive measures and exposure control strategies could be linked to periodic workplace risk assessment of the exposure in the relevant industries. By enhanced follow-up to and use of protective equipment, the burden of fixed AO caused by occupational exposures has the potential to be substantially reduced.