Around 14% of the female European workers <50 years engage in night work (1). Several studies have investigated adverse pregnancy outcomes in relation to work schedules during pregnancy (2–6), but studies focusing on the pregnant women’s health are sparse (7–9).
Hypertensive disorders of pregnancy (HDP) including preeclampsia and gestational hypertension occur in around 8% and 5% of pregnancies worldwide and in Denmark, respectively, and are a major cause of morbidity and mortality (10–12). It is suggested that the incidence of HDP has increased over time probably due to advanced maternal age and increased occurrence of obesity and diabetes in mothers (13, 14). The pathophysiology of HDP is not fully elucidated but seems to involve maternal, fetal and placental factors (14–19).
Night work, including both fixed night shifts and shift work, may influence the risk of HDP in several ways. Psychosocial factors related to night work, such as low job control and work-life conflict, have been associated with cardiovascular diseases including hypertension (20, 21). Another mechanism is through behavioral changes induced by night work affecting sleep, smoking habits, physical activity, diet and body mass (20, 22). Furthermore several physiological mechanisms including circadian disruption, hormonal changes, altered lipids and increased inflammation markers have been proposed linking night work with cardiovascular diseases (20, 22, 23). Melatonin, one of the main hormones affected by circadian disruption, is also produced in the placenta and plays a crucial role in maternal, fetal and placental physiology acting as an anti-inflammatory and immunomodulatory hormone, as well as a regulator of apoptosis (24–32). Furthermore the circadian oscillation of blood pressure is controlled in part by melatonin (33, 34). An altered circadian pattern of blood pressure has been reported in HDP, and as a result melatonin has been studied for its potential use in the treatment of preeclampsia (35, 36).
The few studies that have been conducted on the association between night and shift work with HDP revealed conflicting results (37–40). A major limitation of these studies is the crude assessment of work schedules. For instance in three (37, 38, 40) out of four studies it was not clear whether their definition of shift work included night shifts.
Payroll data provides accurate information on work schedules for a large population overcoming hereby the limitations related to exposure assessment in prior studies (41, 42).
The primary aim of this study was to investigate whether night work expressed by number and duration of night shifts, number of consecutive night shifts and number of quick returns during pregnancy is related to increased risk of HDP. We furthermore investigated whether age, body mass index (BMI) and socioeconomic status (SES) modified the effect of night work on the risk of HDP.
Methods
Design
We conducted a prospective register-based cohort study with information from three Danish national registries linked on individual level through the civil registration number given to all residents in Denmark since 1968.
The Danish Working Hour Database (DWHD), a national payroll database covering more than 250 000 employees in the Danish administrative regions including all hospital employees, provided the source population. It includes daily information on time of start and end of all workdays, sickness absence, paid and unpaid leave, occupation and place of employment from January 2007 to December 2015 (41, 43). Pregnancy information and covariates were identified from the Danish Medical Birth Registry, which contains information from all home and hospital births in Denmark from 1973 onwards (44). Outcome variables were identified from the Danish National Patient Registry, which provides data on inpatients in Danish hospitals since 1977 and on outpatients since 1994 (45).
Study cohort
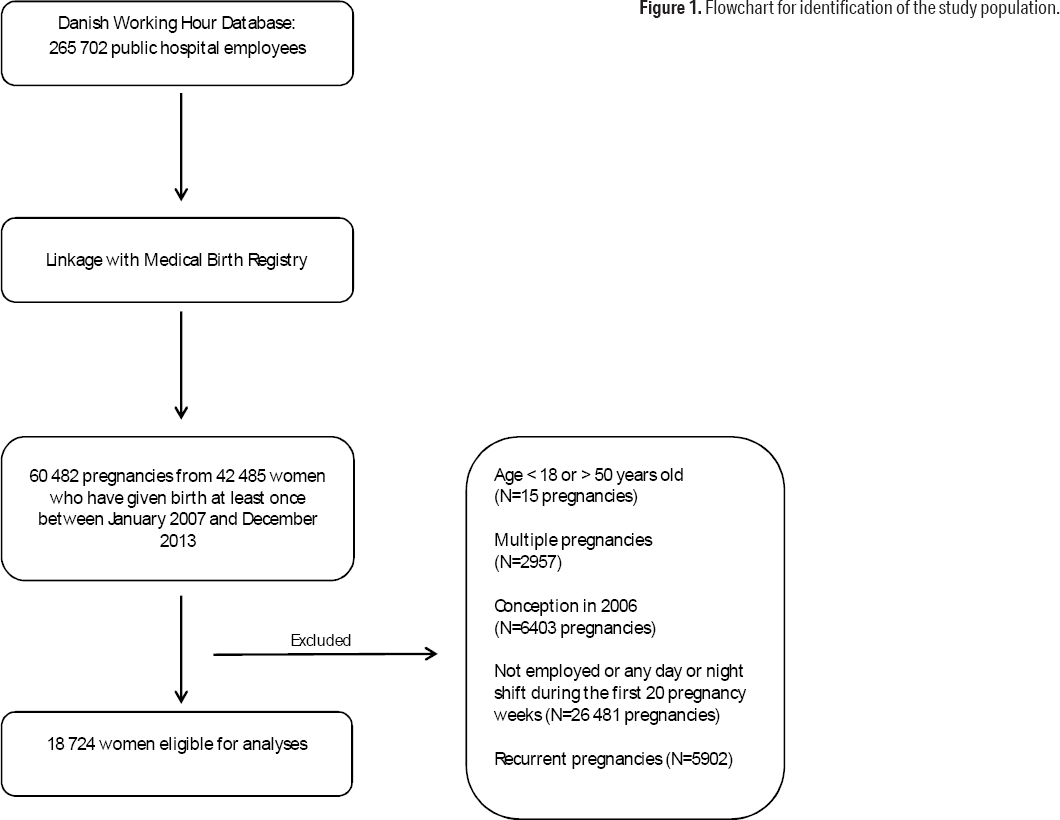
Women from DWHD who gave birth at least once between 2007 and 2013 were identified (N=42 485 women with 60 482 births). We excluded women ≤18 and ≥50 years (N=15), multiple pregnancies (N=2957), pregnancies conceived in 2006 (N=6403), and pregnancies from women without registrations in DWHD of any night or day shift during the first 20 pregnancy weeks (N=26 481). This was the exposure time because gestational hypertension is, by definition, diagnosed after 20 pregnancy weeks (14). To avoid clustering effects, each woman contributed with only one pregnancy, the first during the study period (N=5902 pregnancies excluded), leaving 18 724 women eligible for analyses (figure 1).
Exposure
Exposure definitions are in line with recent studies using payroll data (41, 46).
Shifts, including on-call shifts, lasting ≥3 hours were defined as day (start time after 06:00 and end time before 21:00 hours), evening (end time after 21:00 and before 02:00 hours), night (any start and end time including working hours between 23:00 and 06:00 hours) and early morning (start time between 03:00 and 06:00 hours).
A night worker was defined by working ≥1 night shift and a day worker by working ≥1 day shift but no night, evening or early morning shifts during the first 20 pregnancy weeks.
Consecutive night shifts
Categories of consecutive night shifts were 0 (only single night shifts), 2–3 (at least one spell of 2–3 consecutive night shifts and no spells of ≥4 consecutive night shifts), and ≥4 (at least one spell of ≥4 consecutive night shifts) during the first 20 pregnancy weeks.
Quick returns
We defined quick returns as intervals between shifts lasting <11 hours (47). Quick returns after night shifts were defined as a recovery period of <28 hours after a night shift (46). Categories of number of quick returns and quick returns after night shift were 0, 1–4 and ≥5 quick returns during the first 20 pregnancy weeks.
Covariates
Age (<30, 30–35, >35 years), BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), parity (1, 2, ≥3) and smoking (nonsmoker, former smoker, smoker) registered by the midwife or family doctor at the first antenatal visit were retrieved from the Danish Medical Birth Registry. Classification of SES into high, low or medium was derived from Statistics Denmark. It was based on DISCO-88, the Danish version of the International Standard Classification of Occupations (ISCO-88) (48), in the calendar years 2007–2009, and DISCO-08, the Danish version of ISCO-08 (49), in the calendar years 2010–2013.
Sickness absence three months prior to pregnancy was expressed as the sum of all days registered with ≥3 hours of sickness absence in DWHD during this period. It was categorized as 0, <10 and ≥10 days.
Missing values for parity, smoking and SES represented only 1.4%, 2.9% and 0.2% respectively. Missing values for BMI (4.4%) were evenly distributed across exposure categories. Missing values of sickness absence three months prior to conception (7.9%) occurred when the woman’s employment covered by DWHD had <3 months prior to conception.
Outcome
The outcome of HDP was defined by ICD-10 codes (50): hypertension (I10-15), gestational hypertension (O12, 13, 16) and pre-eclampsia and eclampsia (O14, 15).
Statistical analysis
We computed odds ratios (OR) with 95% confidence intervals (CI) for HDP according to different dimensions of night work during the first 20 weeks of pregnancy by logistic regression. Model 1 refers to crude analyses and model 2 is adjusted for age, BMI, parity, smoking, SES and sickness absence three months prior to pregnancy categorized as described above. Because of too few cases, it was not possible to adjust the analyses for cases of prior HDP (N=287), prior diabetes (N=17), and current gestational diabetes (N=202). Model 3 is further adjusted for number of night shifts in the analyses of consecutive night shifts, quick returns and duration of night shifts.
In all analyses, except for interaction analyses, we made comparisons of night workers with day workers and comparisons within night workers. In the latter, night workers in the lowest category of exposure (1–19 night shifts, duration of night shift of <12 hours, night workers without consecutive night shifts and night workers without quick returns) were used as the reference group.
We investigated whether the association between night work and HDP was modified by age, BMI and SES by a likelihood ratio test comparing models with main effects only with models that in addition included an interaction term, ie, the product of the combined effect. We used a level of significance of 5%.
Gestational length was used to identify conception date. There were only 330 (0.6%) pregnancies with missing values for gestational length but the proportion of still births among these was statistically significant higher (15.4%) than among other pregnancies (0.4%). We therefore substituted the missing values by the mean value of gestational length for live (278 days) and still (220 days) births, respectively.
We performed the following sensitivity analyses: (i) restricted to nulliparous women (N=9 660), (ii) with pre-eclampsia as the outcome (N=18 724), and (iii) restricted to the first trimester as the exposure time (N=18 158). In the latter analysis, night workers had at ≥1 night shift and day workers ≥1 day shift but no night, evening or early morning shifts during the first 12 pregnancy weeks instead of 20 weeks applied in the main analysis.
All analyses were done with the SAS 9.4 software (SAS Institute, Cary, North Carolina, United States).
Results
In our cohort of 18 724 pregnant women, 11 193 were classified as night workers and 7531 as day workers (table 1). The most frequent occupations were nurse (44%), physician (13%), medical secretary (7%), physio/occupational therapist (5%) and laboratory technician (4%) reflecting that the majority of the workers covered by the DWHD are employed at hospitals. Characteristics of day and night workers were rather similar. Day workers had a total of 496 024 day shifts during the first 20 weeks of pregnancy. Night workers had a total of 652 858 shifts being 65% day shifts and 19% night shifts. Only 113 women (1%) worked fixed night shifts. They had higher BMI (mean 25.2 kg/m2, SD 5.0), higher proportion of women with parity ≥3 (27%), higher proportion of current smokers (4.4%) and higher proportion of women with low SES (43%) compared to the other night workers in the cohort. The prevalence of HDP was 3.6% among day workers and 3.8% among night workers.
Table 1
Characteristics of pregnant workers in public administration and hospitals in Denmark, 2007–2013. [SD=standard deviati
| Day work a (N=7531) | Night work b (N=11 193) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| N | % | Mean | SD | N | % | Mean | SD | |
| Age (years) | 31.8 | 4.2 | 30.7 | 3.9 | ||||
| Body mass index (kg/m2) | 24.0 | 7.6 | 23.9 | 7.9 | ||||
| Parity | ||||||||
| 1 | 3462 | 46.0 | 6198 | 55.4 | ||||
| 2 | 2596 | 34.5 | 3156 | 28.2 | ||||
| ≥3 | 1356 | 18.0 | 1701 | 15.2 | ||||
| Smoking | ||||||||
| Non smoker | 6952 | 92.3 | 10 319 | 92.2 | ||||
| Former smoker | 106 | 1.4 | 230 | 2.1 | ||||
| Smoker | 273 | 3.6 | 309 | 2.8 | ||||
| Socioeconomic status | ||||||||
| High | 1844 | 24.5 | 2618 | 23.4 | ||||
| Medium | 3848 | 51.1 | 7575 | 67.7 | ||||
| Low | 1821 | 24.2 | 988 | 8.8 | ||||
| Most frequent c occupations | ||||||||
| Nurse | 1071 | 14.2 | 6,857 | 61.3 | ||||
| Physician | 659 | 8.8 | 2,000 | 17.9 | ||||
| Nurse assistant | 208 | 2.8 | 390 | 3.5 | ||||
| Laboratory technician | 503 | 6.7 | 259 | 2.3 | ||||
| Midwife | 10 | 0.1 | 248 | 2.2 | ||||
| Medical secretary | 1314 | 17.5 | 58 | 0.5 | ||||
| Physio- and ergo therapist | 977 | 13.0 | 30 | 0.3 | ||||
| Cleaning and kitchen staff | 391 | 5.2 | 8 | 0.07 | ||||
| Psychologist | 463 | 6.2 | 1 | 0.01 | ||||
| Shifts during the first 20 weeks of pregnancy | ||||||||
| Day | 65.9 | 23.8 | 37.9 | 17.2 | ||||
| Night | 11.2 | 9.1 | ||||||
| Evening | 9.2 | 9.7 | ||||||
| Early morning | 0.03 | 0.8 | ||||||
| Weekly working hours d | 23.8 | 8.9 | 25.1 | 7.7 | ||||
| Sickness absence days 3 months prior to pregnancy | 2.9 | 7.2 | 2.6 | 5.6 | ||||
Women working ≥1 spell of ≥4 consecutive night shifts during the first 20 pregnancy weeks had higher risk of HDP compared to night workers without consecutive night shifts (OR 1.41, 95% CI 1.01–1.98), see table 2. We furthermore observed a statistically significant trend of increasing risk with increasing number of consecutive night shifts. Women with spells of exclusively 2–3 consecutive night shifts had, on average, 4 consecutive night shifts in total. While women with ≥1 spell of ≥4 consecutive night shifts had, on average, 14 consecutive night shifts in total. Hence these categories express both length of spells and total number of consecutive night shifts.
Table 2
Odds ratios (OR) of hypertensive disorders of pregnancy by consecutive night shifts during the first 20 pregnancy weeks among workers in public administration and hospitals in Denmark, 2007–2013. [CI=confidence interval]
| Consecutive night shifts | Women | Cases | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| All workers (N=18 724) | ||||||||
| Day workc | 7531 | 40.2 | 270 | 3.6 | 1.00 | Referent | 1.00 | Referent |
| 0 | 4003 | 21.4 | 132 | 3.3 | 0.92 | 0.74–1.13 | 0.85 | 0.67–1.08 |
| 2–3 | 5225 | 27.9 | 205 | 3.9 | 1.10 | 0.91–1.32 | 0.97 | 0.79–1.20 |
| ≥4 | 1965 | 10.5 | 89 | 4.5 | 1.28 | 0.99–1.62 | 1.13 | 0.86–1.48 |
| P for trend | 0.05 | 0.62 | ||||||
| Night workers d (N=11 193) | ||||||||
| 0 | 4003 | 35.8 | 132 | 3.3 | 1.00 | Referent | 1.00 | Referent |
| 2–3 | 5225 | 46.7 | 205 | 3.9 | 1.20 | 0.96–1.50 | 1.22 | 0.92–1.62 |
| ≥4 | 1965 | 17.6 | 89 | 4.5 | 1.39 | 1.06–1.83 | 1.41 | 1.01–1.98 |
| P for trend | 0.02 | 0.04 | ||||||
As shown in table 3, we observed a statistically significant trend of increasing risk of HDP with increasing number of quick returns after night shifts. However, the risk estimate for the highest exposed group, those with ≥5 quick returns after a night shift (on average 10.4 quick returns) during the first 20 pregnancy weeks, did not reach statistical significance (OR 1.28, 95% CI 0.87–1.95).
Table 3
Odds ratios (OR) of hypertensive disorders of pregnancy by number of quick returns a and quick returns after a night shift b during the first 20 pregnancy weeks among workers in public administration and hospitals in Denmark, 2007–2013. [CI=confidence interval]
| Women | Cases | Model 1 c | Model 2 d | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| Quick returns | ||||||||
| All workers (N=18 724) | ||||||||
| Day work e | 7531 | 40.2 | 270 | 3.6 | 1.00 | Referent | 1.00 | Referent |
| 0 | 3817 | 20.4 | 128 | 3.4 | 0.93 | 0.75–1.15 | 0.92 | 0.72–1.16 |
| 1–4 | 5123 | 27.4 | 203 | 4.0 | 1.11 | 0.92–1.34 | 1.00 | 0.81–1.23 |
| ≥5 | 2253 | 12.0 | 95 | 4.2 | 1.18 | 0.93–1.50 | 0.94 | 0.72–1.22 |
| P for trend | 0.10 | 0.76 | ||||||
| Night workers f (N=11 193) | ||||||||
| 0 | 3817 | 34.1 | 128 | 3.4 | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 5123 | 45.8 | 203 | 4.0 | 1.19 | 0.95–1.49 | 1.12 | 0.87–1.45 |
| ≥5 | 2253 | 20.1 | 95 | 4.2 | 1.27 | 0.97–1.66 | 1.07 | 0.79–1.46 |
| P for trend | 0.07 | 0.64 | ||||||
| Quick returns after a night shift | ||||||||
| All workers (N=18 724) | ||||||||
| Day work e | 7531 | 40.2 | 270 | 3.6 | 1.00 | Referent | 1.00 | Referent |
| 0 | 1023 | 5.5 | 39 | 3.8 | 1.07 | 0.75–1.48 | 0.84 | 0.55–1.23 |
| 1–4 | 4569 | 24.4 | 160 | 3.5 | 0.98 | 0.80–1.19 | 0.86 | 0.69–1.07 |
| ≥5 | 5601 | 29.9 | 227 | 4.1 | 1.14 | 0.95–1.36 | 1.06 | 0.87–1.29 |
| P for trend | 0.26 | 0.74 | ||||||
| Night workers f (N=11 193) | ||||||||
| 0 | 1023 | 9.1 | 39 | 3.8 | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 4569 | 40.8 | 160 | 3.5 | 0.92 | 0.65–1.33 | 1.03 | 0.69–1.59 |
| ≥5 | 5601 | 50.0 | 227 | 4.1 | 1.07 | 0.76–1.53 | 1.28 | 0.87–1.95 |
| P for trend | 0.30 | 0.05 | ||||||
Table 4 presents the results for long-night-shift workers compared to day workers (OR 1.00, 95% CI 0.81–1.23), and compared to short-night-shift workers (OR 1.08, 95% CI 0.85–1.36). Of all long night shifts, 40% lasted 17–24 hours and 34% lasted 9–16 hours, while 62% of all short night shifts lasted ≤8 hours.
Table 4
Odds ratios (OR) of hypertensive disorders of pregnancy by duration of night shifts during the first 20 pregnancy weeks among workers in public administration and hospitals in Denmark, 2007–2013. [CI=confidence interval]
| Duration of night shifts | Women | Cases | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| All workers (N=18 724) | ||||||||
| Day work c | 7531 | 40.2 | 270 | 3.6 | 1.00 | Referent | 1.00 | Referent |
| <12 hours d | 5734 | 30.6 | 214 | 3.7 | 1.04 | 0.87–1.25 | 0.94 | 0.76–1.16 |
| ≥12 hours e | 5459 | 29.2 | 212 | 3.9 | 1.09 | 0.90–1.31 | 1.00 | 0.81–1.23 |
| P for trend | 0.37 | 0.92 | ||||||
| Night workers f (N=11 193) | ||||||||
| <12 hours | 5734 | 51.2 | 214 | 3.7 | 1.00 | Referent | 1.00 | Referent |
| ≥12 hours | 5459 | 48.8 | 212 | 3.9 | 1.04 | 0.86–1.27 | 1.08 | 0.85–1.36 |
Table 5 presents the results for women who worked ≥20 night shifts during the first 20 pregnancy weeks (on average 28 night shifts) compared to day workers (OR 1.13, 95% CI 0.85–1.48), and compared to women working 1–19 night shifts (OR 1.15, 95% CI 0.86–1.52).
Table 5
Odds ratios (OR) of hypertensive disorders of pregnancy by number of night shifts during the first 20 pregnancy weeks among workers in public administration and hospitals in Denmark, 2007–2013. [CI=confidence interval]
| Number of night shifts | Women | Cases | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| All workers, N=18 724 | ||||||||
| Day work c | 7531 | 40.2 | 270 | 3.6 | 1.00 | Referent | 1.00 | Referent |
| 1–19 | 9560 | 51.1 | 360 | 3.8 | 1.05 | 0.90–1.24 | 0.94 | 0.78–1.12 |
| ≥20 | 1633 | 8.7 | 66 | 4.0 | 1.13 | 0.85–1.48 | 1.09 | 0.81–1.45 |
| P for trend | 0.35 | 0.96 | ||||||
| Night workers d, N=11 193 | ||||||||
| 1–19 | 9560 | 85.4 | 360 | 3.8 | 1.00 | Referent | 1.00 | Referent |
| ≥20 | 1633 | 14.6 | 66 | 4.0 | 1.08 | 0.82–1.40 | 1.15 | 0.86–1.52 |
Further adjustment for number of night shifts (model 3) did not substantially change the results in the analyses of consecutive night shifts, quick returns and duration of night shifts.
The association between night work and HDP was modified by BMI (P-value for multiplicative interaction 0.03). As presented in table 6, analysis among women with BMI ≥30 kg/m2 revealed that those who worked ≥4 consecutive night shifts had substantially increased risk of HPD compared to day workers (OR 5.31, 95% CI 1.98–14.22). The corresponding risk for women with BMI <25 kg/m2 was OR 1.02, 95% CI 0.73–1.41. Further adjustment for BMI among obese women did not change the results. A similar increase was observed for all exposures among obese women (see supplementary tables S1-S4, www.sjweh.fi/show_abstract.php?abstract_id=3728). Due to low statistical power we were unable to make stratified comparisons within night workers only. We found no interaction of any of the analyzed exposures with maternal age or SES.
Table 6
Odds ratios (OR) of hypertensive disorders of pregnancy by consecutive night shifts during the first 20 pregnancy weeks stratified a by body mass index (BMI) among workers in public administration and hospitals in Denmark, 2007–2013. [CI=confidence interval]
| Consecutive night shifts | Women | Cases | Model1 b | Model2 c | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | % | N | % | OR | 95% CI | OR | 95% CI | |
| BMI <25 kg/m2 (N=12 815) | ||||||||
| Day work | 5119 | 40.0 | 201 | 3.9 | 1.00 | Referent | 1.00 | Referent |
| 0 | 2952 | 23.0 | 96 | 3.3 | 0.82 | 0.64–1.05 | 0.77 | 0.59–1.02 |
| 2–3 | 3545 | 27.7 | 154 | 4.3 | 1.11 | 0.90–1.38 | 0.95 | 0.75–1.21 |
| ≥4 | 1199 | 9.4 | 56 | 4.7 | 1.20 | 0.89–1.62 | 1.02 | 0.73–1.41 |
| BMI 25–29 kg/m2 ( N=3501) | ||||||||
| Day work | 1419 | 40.5 | 52 | 3.7 | 1.00 | Referent | 1.00 | Referent |
| 0 | 644 | 18.4 | 24 | 3.7 | 1.02 | 0.62–1.67 | 0.93 | 0.55–1.56 |
| 2–3 | 994 | 28.4 | 40 | 4.0 | 1.10 | 0.72–1.68 | 0.97 | 0.63–1.51 |
| ≥4 | 444 | 12.7 | 16 | 3.6 | 0.98 | 0.56–1.74 | 0.94 | 0.53–1.69 |
| BMI ≥ 30 kg/m2 (N=1588) | ||||||||
| Day work | 671 | 42.3 | 7 | 1.0 | 1.00 | Referent | 1.00 | Referent |
| 0 | 229 | 14.4 | 10 | 4.4 | 4.33 | 1.63–11.52 | 3.47 | 1.15–10.52 |
| 2–3 | 435 | 27.4 | 8 | 1.8 | 1.78 | 0.64–4.94 | 1.60 | 0.53–4.83 |
| ≥4 | 253 | 15.9 | 13 | 5.1 | 5.14 | 2.03–13.03 | 5.31 | 1.98–14.22 |
Overall sensitivity analyses slightly attenuated the estimates across all exposures. The effect of consecutive night shifts during the first 20 pregnancy weeks was consistent although not statistically significant throughout sensitivity analyses within night workers (OR 1.39, 95% CI 0.94–2.05 restricted to nulliparous women, OR 1.40, 95% CI 0.91–2.15 with pre-eclampsia as the outcome, and OR 1.36, 95% CI 0.96–1.93 with the first trimester as the exposure time). Regarding the question on possible selection out of night work during pregnancy, we identified only 580 women (5%) who worked at least one night shift during the first trimester and changed to fixed day work during the second trimester. These women had similar age (mean 31 years), BMI (mean 23.7 kg/m2) and smoking habits (2.8% current smokers) as the rest of the cohort but presented a higher proportion of physicians (37%).
Discussion
To our knowledge, this is the first study to investigate the association between HDP with different dimensions of night work objectively assessed through payroll data. In our study, workers with ≥4 consecutive night shifts during the first 20 pregnancy weeks had higher risk of HDP compared to night workers without consecutive night shifts (OR 1.41, 95% CI 1.01–1.98). We furthermore observed a dose−response gradient for number of consecutive night shifts and the risk of HDP. The fact that this effect was observed in comparisons within night workers strengthens the evidence of a causal effect as the group of night workers is more homogeneous. These analyses may therefore be less susceptible to the healthy worker effect present in comparisons of night versus day workers. In fact, we observed higher risk estimates in comparisons within night workers for all the exposures. Comparisons within night workers may be more appropriate from an epidemiological point of view. On the other hand, analyses restricted to night workers exclude an unexposed group and some selection bias regarding different dimensions of night work remains. Previous studies have shown that individual preferences related to both personal (chronotype, sleep flexibility, social context) (51–53) and occupational (work content, demands and environment) (54) factors vary substantially among night workers resulting in differences in adaptation to night work. Accordingly we found that workers with fixed night work during the first 20 pregnancy weeks differed in BMI, parity, smoking habits and SES compared to the other night workers in the cohort. Compared to the background Danish population, our cohort presented lower prevalence of smoking during pregnancy (3% versus 12%) (55) and lower proportion of overweight women (19% versus 46%) (56), which may reflect a more health promoting behavior among healthcare professionals.
Our findings are in accordance with recent studies focusing on consecutive night shifts rather than solely on the number of night shifts. For example increasing the number of consecutive night shifts has been associated with progressive changes in hormones involved in circadian regulation, such as melatonin, cortisol, thyroxin and prolactin (30, 31, 57). Such changes have been observed down to three consecutive night shifts (58, 59). Furthermore, it has been suggested that at least two days off work are required to allow for circadian readjustment following 2–4 consecutive night shifts (31, 60). In our cohort, the majority of hospital employees had rotating shifts with different schedules nearly every week which do not fulfill this recommendation. Hence, in this context, working consecutive night shifts may lead both to circadian disruption and to insufficient recovery. Our findings of increasing odds ratios of HDP with increasing number of quick returns after night shifts also support the potential effect of insufficient recovery after a night shift.
In our data, BMI modified the effect of night work on the risk of HDP, as obese women who worked longer night shifts, longer spells of consecutive night shifts and had the highest number of quick returns after night shifts had 4–5 fold increased risk of HDP compared to day workers. It is known that pre-pregnancy BMI is an important risk factor for HDP independent of weight gain during pregnancy (17, 61, 62). Even though these results are based on few cases, they are consistent across exposures. Obese women neither had higher proportion of workers with fixed night shifts nor a gradient of increasing BMI from day to night workers.
We hypothesized that women who worked night shifts during the first trimester and changed working schedule to only day work during the second trimester due to health problems might cause bias towards the null as the exposure time in the main analysis was 20 weeks. However, sensitivity analysis resetting exposure time to the first 12 pregnancy weeks indicated no such bias. On the other hand, analysis restricted to the first trimester excludes a possible effect of night work during the second trimester, which may in part explain the attenuation of the estimates. Even though the physiopathology of HDP seems to be related with placenta development in the beginning of pregnancy (14), demographic and lifestyle factors on the second and third trimester of pregnancy seem also to influence the risk of HDP (63).
We found no statistically significant association between HDP with any of the analyzed dimensions of night work compared to day workers, suggesting that the effect of night work on the risk of HDP is related to the way night shifts are organized rather than the mere presence of night shifts. This can be in part due to differences in work content and work environment between day and night workers. We did not observe the presence of more pronounced risk factors for HDP among night workers compared to day workers. Actually our cohort of night workers had lower BMI, a lower proportion of smokers and a lower proportion of workers with low SES than day workers. Similar to our results, three out of four previous studies that compared shift workers with day workers found no association between with HDP (37, 39, 40). Wergeland & Strand (38) reported an increased prevalence of pre-eclampsia among shift workers, but only among parous women.
The main strengths of our study are the large and national sample size, the objective and detailed exposure assessment, and the use of validated and objective registries for identification of covariates and outcomes, which makes information bias and selection in and out of the study unlikely. Furthermore, we evaluated different dimensions of night work within night workers and restricted the exposure time to specific periods of pregnancy. Some limitations include a lack of information on workload during night shifts, such as the possibility for sleep during on call shifts, and on chronotype and personal preferences of the participants. The latter is especially relevant because night work is compulsory for the majority of occupations in our cohort. Additionally, our study design did not account for the healthy worker effect, where women with health problems in general tend to choose day work. As our cohort comprises primarily healthcare professionals, our results may not apply for pregnant workers in other occupations.
Ideally future studies on health effects of night work during pregnancy should combine objectively assessed work schedules with information on chronotype and personal preferences, work content and environment, and should perform comparisons both with day workers and within groups of night workers.
Concluding remarks
In this nationwide study of Danish pregnant workers in the public health sector with objectively assessed work schedules, working consecutive night shifts and quick returns after night shifts during the first 20 pregnancy weeks was associated with an increased risk of HDP, in particular among obese women. Possible ways for avoiding such risk when organizing night work during pregnancy are favoring single night shifts or short spells of consecutive night shifts and reducing quick returns by allowing for adequate recovery time following night shifts.