Medical personnel in specialized wards may be exposed to various genotoxic and carcinogenic agents, including volatile anesthetics, antineoplastic agents, and formaldehyde. The mutagenic and carcinogenic effects of these agents have been frequently discussed (1–13) as they pose a potential genotoxic burden and health problems for a considerable number of individuals in various branches of medicine. Consequently, a conclusive assessment of chromosomal damage among medical personnel is of particular importance.
Anesthesiologists form an inseparable part of operation teams with occupational exposure to volatile anesthetics. Presently, the most frequently used anesthetics include sevoflurane, isoflurane, desflurane, and entrane, all of which have gradually replaced the more toxic halothane. Describing isoflurane- and halothane-induced elevation of DNA damage and degradation due to cell death in peripheral human lymphocytes (PBL) in vitro, the study of Jaloszynski et al (11) is one of the first on the possible genotoxicity of anesthetics. Cellular DNA damage increased after exposure to both desflurane and halothane (12). In the recent study of Rozgaj et al (14), increased levels of DNA damage were reported among anesthesiologists exposed to the anesthetic gases sevoflurane, isoflurane, and nitrous oxide. The authors also recorded a three-fold increase in the frequency of micronuclei, representing chromosomal damage (14). These findings are in accordance with the earlier data of Lewinska et al (15), who demonstrated a significant increase in micronucleus frequency in PBL among nurses exposed to nitrous oxide. A putative mode of action of isoflurane could be the formation of reactive trifluoroacetyl ester, resulting in modification of N7-position of guanine. However, induction of oxidative DNA damage has also been postulated for isoflurane, sevoflurane, and nitrous oxide (16–18).
Although the hazard is well known, employees in oncological units may experience occupational exposure to low doses of antineoplastic agents. The majority of cytostatics are genotoxic, either directly or following biotransformation into electrophilic intermediates (19), or damage the genetic material by secondary mechanisms. The International Agency for Research on Cancer (IARC) has classified many antineoplastic agents as class 1 human carcinogens (eg, cyclophosphamide, etoposide, busulfan, melfalan), class 2A probably carcinogenic to humans (eg, azacitidine, cisplatin, doxorubicine) or class 2B possibly carcinogenic to humans (bleomycin, dacarbazine, mitoxantrone, mitomycin) (20). Occupational exposure to antineoplastic agents has been shown to result in an increased frequency of total chromosomal aberrations (CATot) in PBL, as documented by a number of studies (2, 21–26). Interestingly, nurses occupationally exposed to antineoplastic drugs showed an elevated level of not only chromatid-type aberrations (CTA) (induced by most chemical clastogens), but also chromosome-type aberrations (CSA), such as chromosome breaks and dicentric chromosomes, which is typical of radiation exposure (23).
Formaldehyde is used in histopathology laboratories worldwide as a fixative and tissue preservative. Although the IARC has classified formaldehyde as a class 1 carcinogen (13), only a few studies have explored its genotoxic effects in expected target organs, ie, upper respiratory tract (27). However, significant associations between occupational exposure to formaldehyde and the frequencies of CATot, sister chromatid exchanges (SCE) and micronuclei in PBL of formaldehyde-exposed individuals have been reported (28). Despite the fact that only scant concentration of formaldehyde reaches the blood cells, long-term, cumulative occupational exposure may underlie the cytogenetic effects of formaldehyde.
In this study, we quantified the association between the occupational exposure of physicians and nurses employed in operating rooms (exposure to volatile anesthetics), oncologic units (exposure to antineoplastic agents), and pathological departments (exposure to formaldehyde) and the occurrence of chromosomal aberrations (CA) compared with sex- and age-matched controls. This is an important issue, since the above-mentioned compounds are classified as proven (formaldehyde and cyclophosphamide, for instance) or potential human carcinogens (13, 29, 30) and the level of CA is considered to be predictive of cancer risk (31–33).
Methods
Study population
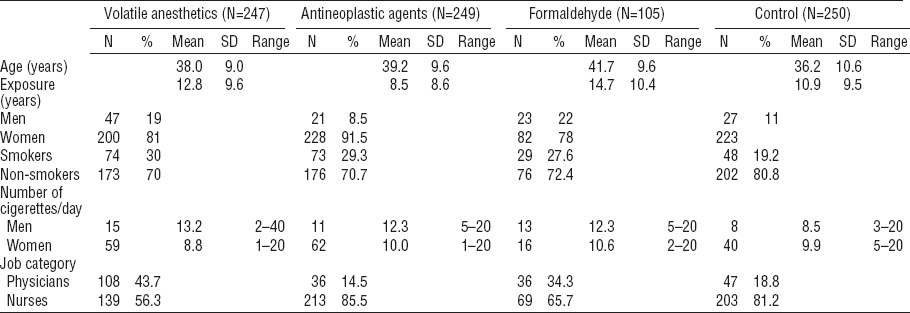
The subjects were recruited in a university hospital, a central military hospital, a faculty hospital and seven regional hospitals, all located in Central Slovakia. Table 1 summarizes the study subjects’ characteristics, including main confounders. Briefly, the studied medical personnel consisted of anesthesiologists occupationally exposed to volatile anesthetics in operating rooms during the administration of volatile anesthetics as a part of complete anesthesia (N=247), staff from specialized oncologic units exposed to antineoplastic agents (N=249), and pathologists from pathologic anatomy departments (N=105). The control group consisted of healthy medical staff from the university hospital and central military hospital with no apparent exposure to genotoxic agents (N=250). The group of anesthesiologists consisted of 139 anesthesiological nurses and 108 physicians-anesthesiologists, the staff from oncology units comprised 213 nurses (including 12 technicians working with cytostatic waste) and 36 physicians, and the departments of pathologic anatomy employees totaled 69 laboratory technicians and 36 pathologists. Blood sampling and data collections were conducted during 2006–2011. Only those subjects apparently healthy at the time of sampling were enrolled in the study. Additionally, individuals with close relatives with any malignant diseases were excluded. Otherwise, no other exclusion criteria were imposed. There was no statistical difference in age between the genders.
Table 1
Characteristics of medical workers occupationally exposed to volatile anesthetics, antineoplastic agents, and formaldehyde compared to unexposed control individuals.
All individuals studied completed a questionnaire regarding the mode and duration of exposure, job category, various exogenous factors (such as smoking, drug usage, exposure to X-ray radiation, alcohol consumption, and dietary habits) prior to blood collection and provided a written consent to be included into the study. The present study adheres to all principles of personnel data protection and was conducted according to the Helsinki Declaration. The local Ethical Committee of the Jessenius Medical Faculty approved the study design.
Estimation of the exposure
Exposure to volatile anesthetics was estimated by ambient air measurements in operating rooms of a hospital. The permissible average exposure limit (PEL) of sevoflurane and isoflurane is 10 ppm (80 mg/m3), with a short-term concentration not exceeding 20 ppm (150 mg/m3). The average determined concentration of sevoflurane and isoflurane (based on 2 measurements at 24 different places in various operating rooms at the University Hospital) was 200.3 mg/m3 (range 64.2–483.9 mg/m3). During the administration of volatile anesthetics (as a part of complete anesthesia in operating rooms), the nominal amounts of 150 l of sevoflurane and 2 l of isoflurane were used in 2011. Exposure to >40 antineoplastic agents could be estimated only on the basis of annual use of active compounds in 2011; the most frequently used were cyclophosphamide (930 g), cytarabin (520 g), rituximab (420 g), fluoruracil (270 g), and ifosfamide (270 g). The medical staff wore protective clothing and gloves, and the preparation of cytostatics took place in laminary flow or fume hoods. The concentration of formaldehyde at the department of pathologic anatomy, measured once a year, was 0.32 mg/m3 (range 0.14–0.66 mg/m3), which is below the maximum accepted ambient air concentrations of formaldehyde in Slovakia (average concentration of 0.37 mg/m3, short-term concentration 0.74 mg/m3).
Cytogenetic analysis
The peripheral blood sampling by venepuncture was performed as a part of specialized medical examinations between 2009–2011. The methodology for cytogenetic analysis has been described earlier (33, 34) and was in accordance with existing guidelines (35). Briefly, peripheral blood lymphocytes stimulated to grow by phytohemagglutinin were cultured for 48 hours. Two microscopists conducted microscopical analysis (each evaluating half of the 100 mitoses scored per subject) in a double-blind fashion on coded slides (codes were broken only after the end of the analysis of the whole subgroup/hospital). The frequency of CATot and the constituent CTA and CSA were evaluated. Inter- and intra-arm exchanges of CTA and CSA (mainly dicentrics and centric rings) were also analyzed (36).
Statistical analysis
Univariate statistics for the cytogenetic endpoints of interest (CATot, CTA, and CSA) and selected covariates were calculated. Furthermore, histograms to describe the distributions of CATot, CTA, and CSA were formulated. Differences in the frequencies of CA among the different occupational groups were tested by non-parametric Mann-Whitney U-test and Kruskal-Wallis test.
In the next step, the subjects were classified according to the median of CATot distribution as either low (frequency <2%) or high (frequency ≥2%). This arbitrary cut-off point was chosen on the basis of long-term experience with this kind of biological monitoring in the Czech and Slovak Republics (37). Regarding CTA and CSA, the subjects were classified as having low (<1%) or high (≥1%) frequency, and, in the case of chromatid and chromosome exchanges, the subjects were classified according to the presence or absence of these chromosomal categories. Stratified data analysis was employed to evaluate relationships between frequencies of categorized cytogenetic endpoints and variables describing occupational exposures and potential confounders, such as job category (1=physicians, 0=nurses), smoking (1=yes, 0=no) and gender (1=man, 0=women). Crude odds ratios (OR) and OR adjusted for gender, job, and smoking specific strata are reported with 95% confidence intervals (95% CI). The differences between the frequencies of evaluated cytogenetic endpoints were tested by Chi-square test. The adjusted OR were calculated by binary logistic regression where gender, job, smoking, and age (calendar years) of the subjects were included as variables.
The statistical analysis was performed by SPSS analytical package version 16.0 (SPSS Inc, Chicago, IL, USA) and SAS JMP 8 (SAS Institute, Cary, NC, USA).
Results
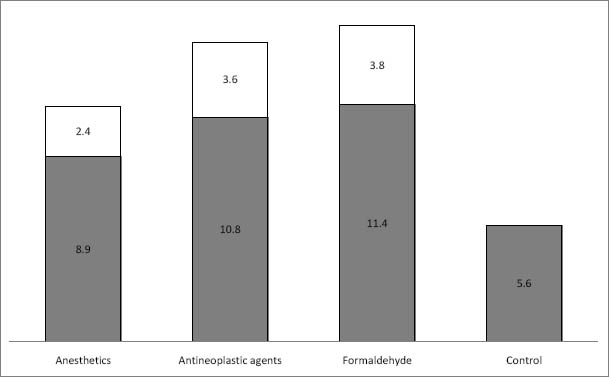
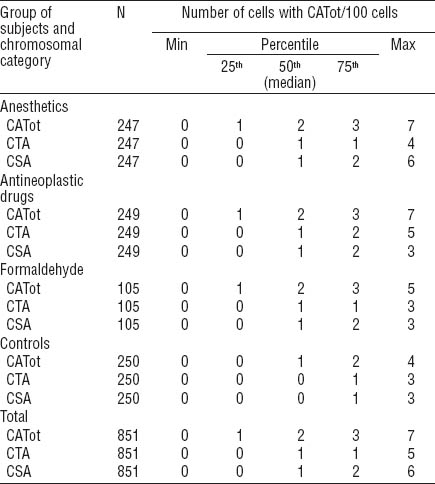
The distributions of CATot, CTA, and CSA among all individuals investigated are presented in figures 1A, B and C. We observed significantly higher frequencies of CATot, CTA, and CSA in all groups of medical workers with occupational exposure as compared to the unexposed medical employees, and these differences were also seen in the distributions of the cells with CATot, CTA, and CSA (Figure 1a, b and c, respectively; P<0.0001 by Kruskal-Wallis). Table 2 presents the median, minimal, and maximal values and percentiles of the evaluated cytogenetic endpoints. Significantly higher frequencies of CATot were separately observed in all exposed groups in comparison with the controls (P<0.0001, Mann-Whitney U-test). CTA and CSA were higher in subjects exposed to volatile anesthetics (P<0.001 and P<0.0001, respectively, Mann-Whitney U-test), cytostatics (P<0.0001 and P<0.0001, respectively, Mann-Whitney U-test), and formaldehyde (P<0.01and P<0.01, respectively, Mann-Whitney U-test) as compared to the control subjects.
Figure 1
Distribution of the number of cells with total chromosomal aberrations (CATot), chromatid-type aberrations (CTA), and chromosome-type aberrations (CSA) in peripheral blood lymphocytes of the exposed (upper diagram) and control (lower diagram) individuals investigated.
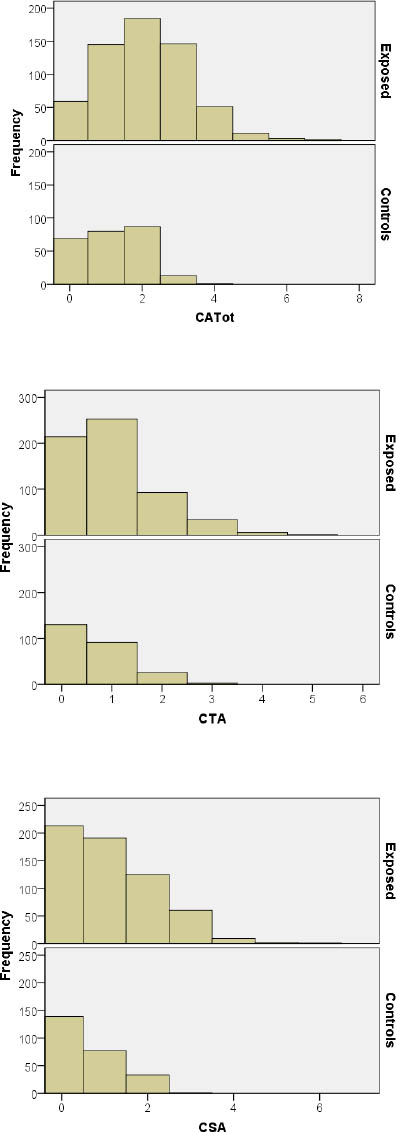
Table 2
Median (50th percentile), interquartile range (25th and 75th percentiles), and minimum and maximum values of distributions of the number of cells (per 100 cells) with any type of total chromosomal aberrations (CATot), chromatid-type aberrations (CTA), and chromosome-type aberrations (CSA) among medical workers occupationally exposed to volatile anesthetics, antineoplastic agents, and formaldehyde compared to unexposed control individuals.
When we analyzed the categorized frequencies of CA, the stratified analyses revealed that there were generally consistent effects of occupational exposures over the strata. The few inconsistencies observed could be explained by the lack of data in some strata. The stratified analyses, therefore, did not suggest the presence of biologically significant interactions. Furthermore, the comparison of the adjusted and crude estimates of OR did not suggest the presence of a confounding effect and it may be concluded that job, smoking, gender, and age were not important confounders in any presented model.
When we focused on the binary logistic models presented in tables 3A, B, and C, we observed that the risk of increased frequency of CATot was associated with occupational exposure to anesthetics (OR 3.9, 95% CI 2.7–5.8), cytostatics (OR 2.7, 95% CI 1.9–3.9), and formaldehyde (OR 1.7, 95% CI 1.1–2.7). No other covariate contributed significantly to the model. When we analyzed the effects of the variables studied on the categorized frequency of CTA, we observed that this cytogenetic endpoint was associated with exposure to anesthetics (OR 1.8, 95% CI 1.3–2.6) and cytostatics (OR 2.6, 95% CI 1.8–3.8). No other variable contributed significantly to this model. A similar finding was also recorded for CSA. We observed significant associations between CSA and anesthetics (OR 3.8, 95% CI 2.5–5.6) and cytostatics (OR 1.7, 95% CI 1.2–2.4). The association between CSA and formaldehyde (OR 1.6, 95% CI 1.0–2.5) was of borderline significance. Lack of data on chromatid exchanges prevented us from similar analyses on this rare aberration category. However, in the case of chromosomal exchanges, we found an association with formaldehyde (OR 2.6, 95% CI 1.1–5.9). Analogically to other models, no other covariate contributed significantly to this model.
Table 3A
Binary logistic regression to describe relative risk of chromosomal aberrations (CA) for particular exposures in medical professions studied – stratified analysis for main confounders. Due to the low number of subjects, stratified results are not shown for smoking female physicians and for non-smoking or smoking male nurses. [OR=odds ratio; 95% CI=95% confidence interval.]
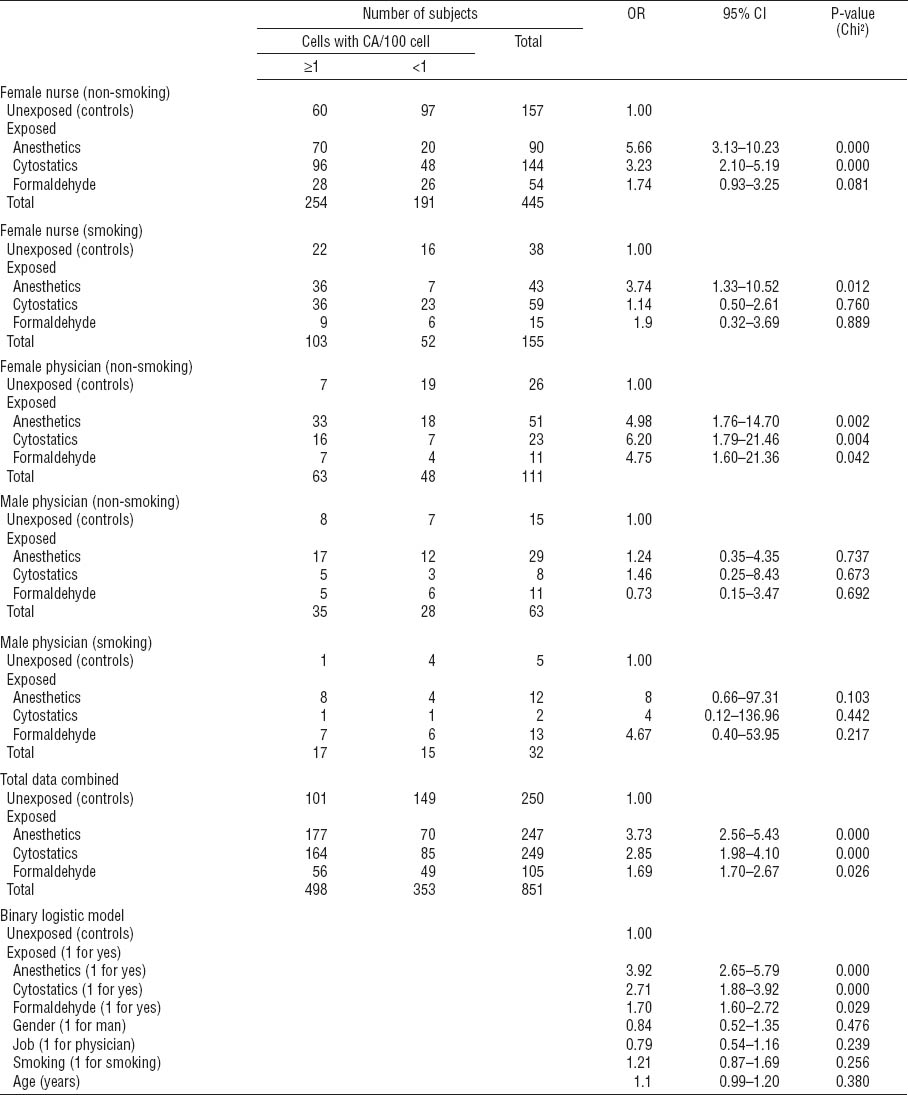
Closer investigation of the distribution of CATot within the strata revealed that a significant effect of occupational exposure to anesthetics, cytostatics, and partially formaldehyde (for female physicians) emerged among non-smoking female nurses and physicians (table 3A); exposure to anesthetics also increased CATot among smoking female nurses. There were not enough male nurses to perform a proper evaluation, but male physicians did not show exposure-related effects. We found a higher median value for CATot among smoking controls than non-smokers, but this difference was not significant. Among exposed individuals, no effect of smoking was apparent (data not shown). Various other exogenous factors (such as drug usage, exposure to X-rays or other types of ionizing radiation, alcohol consumption, and dietary habits) were not significantly different in the exposed groups as compared to the controls; thus they did not modulate the frequency of CATot (data not shown).
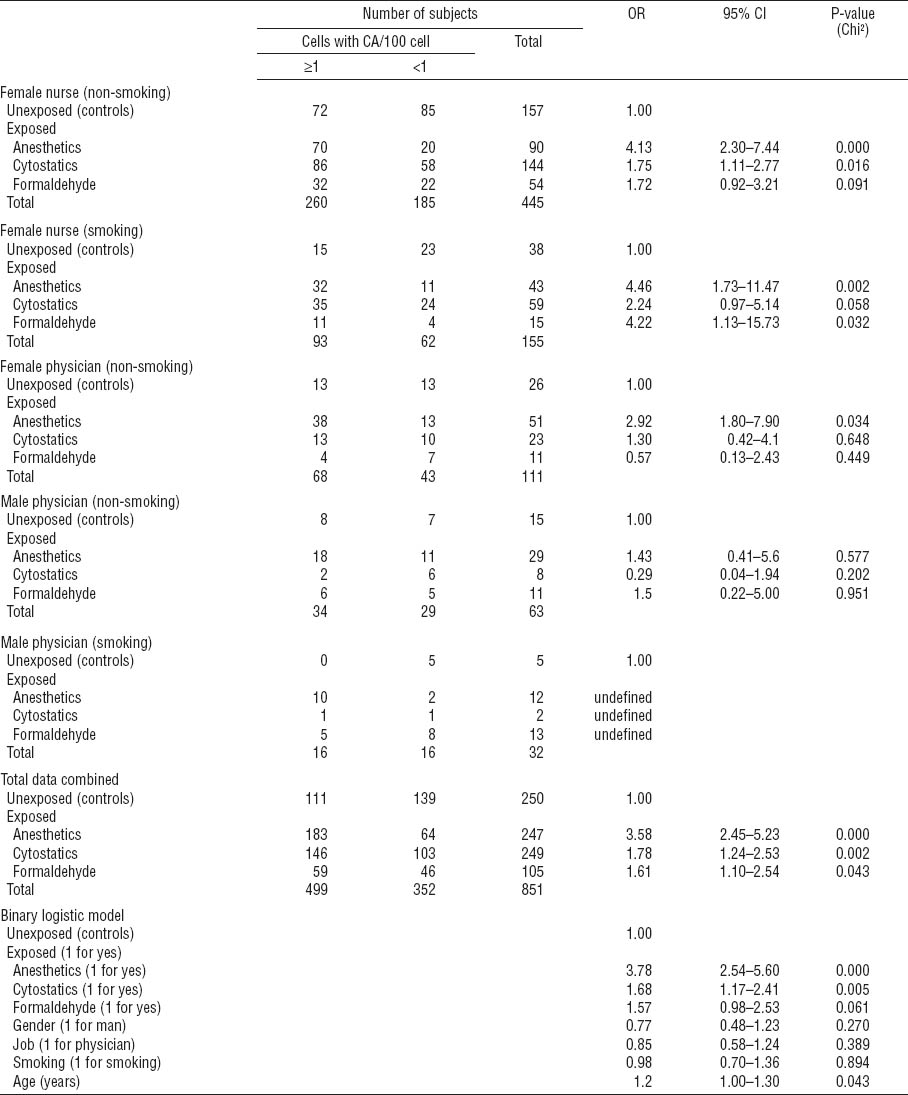
Stratified data analysis for CTA revealed significantly increased OR in association with exposure to anesthetics and cytostatics only among non-smoking female nurses (OR 3.0, 95% CI 1.7–5.2 and OR 2.8, 95% CI 1.7–4.5, respectively; table 3B). Stratified analysis for CSA (table 3C) revealed a significant association with exposure to anesthetics and cytostatics among non-smoking female nurses (OR 4.1, 95% CI 2.3–7.4 and OR 1.8, 95% CI 1.1–2.8, respectively) and non-smoking female physicians exposed to anesthetics (OR 2.9, 95% CI 1.1–7.9). Interestingly, the frequency of CSA was significantly associated with exposure to anesthetics also among smoking female nurses (OR 4.5, 95% CI 1.7–11.5). A significant outcome for formaldehyde in the same group appeared less reliable due to the low number of subjects investigated (OR 4.2, 95% CI 1.1–15.7).
Table 3B
Binary logistic regression to describe relative risk of chromatid-type aberrations (CTA) for particular exposures in medical professions studied – stratified analysis for main confounders. Due to the low number of subjects, stratified results are not shown for smoking female physicians and for non-smoking or smoking male nurses. [OR=odds ratio; 95% CI=95% confidence interval.]
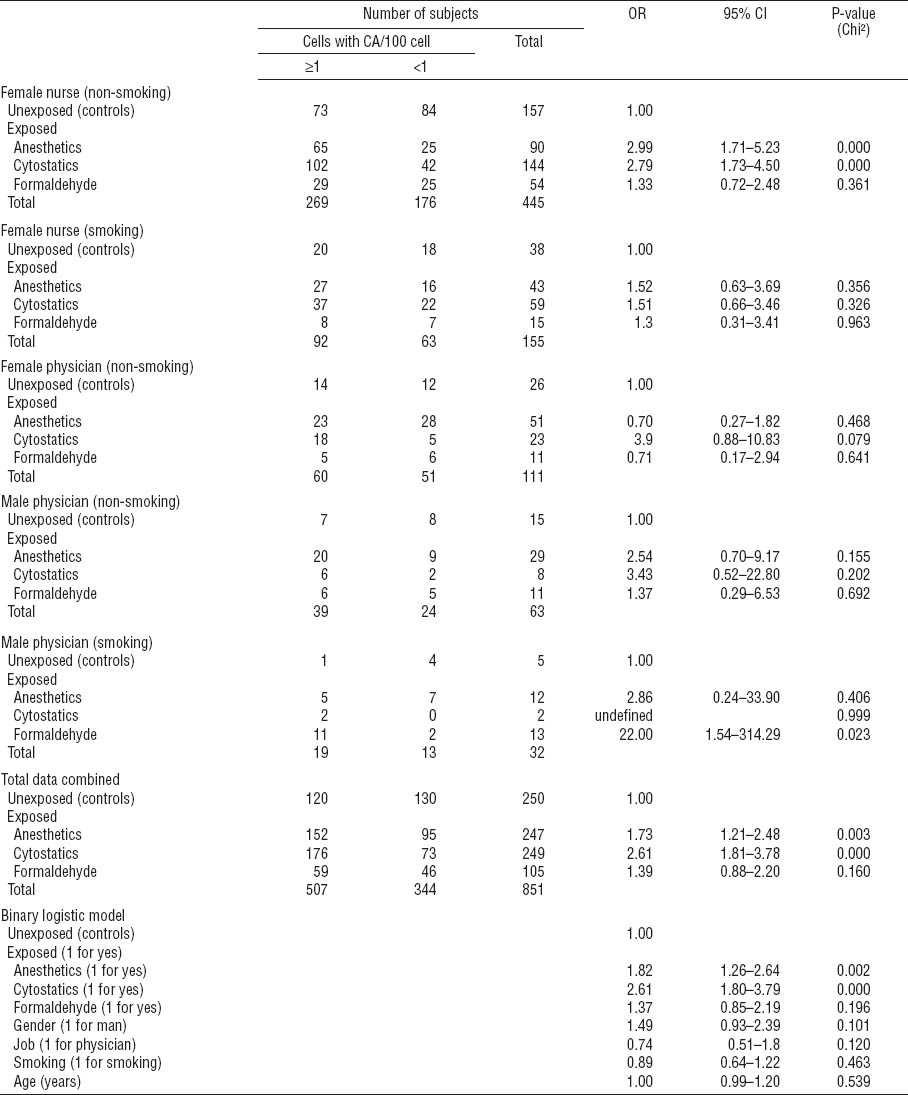
Table 3C
Binary logistic regression to describe relative risk of chromosome-type aberrations (CSA) for particular exposures in medical professions studied – stratified analysis for main confounders. Due to the low number of subjects, stratified results are not shown for smoking female physicians and for non-smoking or smoking male nurses. [OR=odds ratio; 95% CI=95% confidence interval]
An analysis of chromatid and chromosome inter-arm and intra-arm exchanges revealed that the percentage of subjects with exchanges, irrespective of their type, was significantly higher in all the exposed groups than controls (figure 2). Generally, chromatid-type exchanges were substantially less abundant than chromosome-type exchanges which included mainly dicentrics and centric rings. As apparent from figure 2, the highest proportion of both chromatid- and chromosome-type exchanges was recorded among individuals exposed to formaldehyde. This observation was further supported by binary logistic regression, where chromosome-type exchanges were associated with exposure to formaldehyde (OR 2.6, 95% CI 1.1–5.9; P=0.024, χ2 test) irrespective of any confounder recorded.
Discussion
In our study, we report significantly increased chromosomal damage among medical staff occupationally exposed to various genotoxic compounds in specialized hospital wards, comprising several medical professions. It is noteworthy that potentially harmful exposures in medical care may concern relatively large teams and, thus, represent a considerable health risk. The first at risk are anesthesiology workers exposed to volatile anesthetics. Several papers have reported an increase of CATot among operating rooms workers (38–40), and the present study, based on a large group of 247 subjects, strongly supports the earlier findings. The increased CA frequency may be caused by an accumulation of volatile anesthetics in operating rooms, due to inappropriate conditions such as ineffective or inadequate air circulation and ventilation. Epidemiologic studies have disclosed markedly higher concentrations of anesthetics in operating rooms without effective air circulation (41). More than a ten-fold hourly exchange of air in operating rooms resulted in a significant decrease of halothane concentration (42). According to Slovak law, the average workplace air concentration of anesthetics should not exceed 80 mg/m3 (10 ppm), and short-term concentrations should not exceed 20 ppm (150 mg/m3). In the anesthesiology units included in the present study, the concentrations of anesthetics (sevoflurane in particular) in operating rooms were assessed during 2011, and the average concentration of sevoflurane was found to be more than two-fold higher than the limit (ranging from 64.2–483.9 mg/m3 with an average of 200.3 mg/m3 or 25 ppm).
In the present study, the CATot frequencies in the control population were in a range similar to what we described earlier (43). Exposures to anesthetics and cytostatics were associated with an increase in both CTA and CSA. For cytostatics, the induction of CTA was expected because many of the anti-cancer drugs used were S-dependent clastogens (cyclophosphamide and ifosfamide) (44, 45). The increase in CSA observed in the cytostatics exposure group may not primarily have been due to true radiomimetic drugs, since bleomycin was only marginally used, but could rather have reflected derived-type CSA formed from CTA that had occurred in an earlier division in vivo or effects of agents able to prevent G1 DNA repair (cytarabine). The possible genotoxic mechanisms of anesthetics are poorly understood, although oxidative DNA damage (isoflurane, sevoflurane, and nitrous oxide) and modification of N7-position of guanine (isoflurane) have been suggested (16–18). The present study assessed the clastogenic effects of chronic exposures to mixtures of chemicals with different modes of action, and very little is known about the effects of such exposures on peripheral T-lymphocytes with variable half lives in the body.
The higher frequencies of chromosomal damage among female compared to male physicians were possibly associated with higher exposures of women to the individual genotoxic contaminants in the hospital environment, as apparent from the stratified data analysis. There was no general difference between the sexes for any type of CATot, in accordance with earlier studies (34, 44, 45), in contrast to micronuclei which showed a higher rate among women in a number of studies (44).
Most antineoplastic agents exhibit genotoxic, mutagenic, or carcinogenic effects (30). They produce irregular cell division, damage intercellular surroundings, and induce cellular death. Increased frequencies of CATot, SCE, and micronuclei after occupational exposure to antineoplastic agents have been previously reported (1, 21, 46, 47). However, these studies have been conducted on a limited number of subjects. Chromosomal damage related to exposure to antineoplastic drugs may also arise as a consequence of secondary oxidative stress (48). Several reports investigated the extent of chromosomal damage or other types of genotoxic effects in association with various routes of exposure, such as inhalation or skin absorption, with and without the use of protective means (49–51). Moretti et al (52) recently proposed a protocol for epidemiological studies by using an integrated environmental and biological monitoring approach for investigating the genotoxic effects of antineoplastic drugs. Although our study, reporting elevated chromosomal damage among 249 nurses and physicians, provides robust and convincing evidence for health risks in oncology units, individual exposure to cytostatics could not be determined and only information on the total use of cytostatics in 2011 (cyclophosphamide 930 g, cytarabine 520 g, rituximab 420 g, fluoruracil 270 g, and ifosfamide 270 g) were available.
Although formaldehyde is a proven chemical carcinogen (13), it is still frequently used in histopathology laboratories worldwide as a fixative and tissue preservative. Occupational exposure to formaldehyde was found to increase the frequency of CATot, SCE, and micronuclei in PBL in a number of studies (3, 6, 8–10), probably due to a long-term effect of the low doses that reach blood cells. Despite the fact that formaldehyde exposure was associated with lower frequencies of CATot, CTA, and CSA than exposure to anesthetics and antineoplastic drugs in our population, formaldehyde represents a serious health risk particularly as the frequencies of chromatid and chromosome exchanges were three-fold higher among the formaldehyde-exposed workers than the controls. Formaldehyde concentration at the department of pathologic anatomy, measured once a year, was 0.32 mg/m3 (range 0.14–0.66 mg/m3), which is below the Slovakian PEL for formaldehyde (average concentration of 0.37 mg/m3, short-term concentration 0.74 mg/m3).
Regarding the effect of main confounders on the level of chromosomal damage, higher frequencies of total CATot as well as CSA were found among female nurses compared to medical doctors. A similar finding has been published earlier (53). The explanation for the difference in chromosomal damage between the nurses and the doctors may be the longer and higher exposure of the former group; however, personal dosimetry was not available to discern if this was the true reason. We observed a slightly increased frequency of CATot among smokers, which did not reach statistical significance; this potential confounder did not contribute to the presented binary logistic models. The small influence of smoking on CATot was only apparent among the control individuals but not the exposed as if the effect appeared to be masked by the occupational exposure.
Concluding remarks
Our findings indicate that the presence of genotoxic compounds in operating rooms, oncological units, and pathological departments results in a significant increase of chromosomal damage (impairment of chromosomal integrity) among the medical workers employed in these facilities. Since CA are considered predictive of cancer risk, effective measures aimed at the reduction of exposures should be imposed.
Further studies should focus on the assessment of the relationship between individual exposure and risk among the medical personnel and the identification of the reason for the increased level of CATot among females. Additional information about DNA damage that could be associated with CA, and about individual ability to deal with this damage, could be obtained by determining DNA damage markers, such as DNA adducts or DNA double-strand breaks, and DNA repair capacity (54). Such studies may help in understanding the genotoxic burden present in the hospital environment.