Upper-extremity musculoskeletal disorders (MSD) affecting the soft tissues of the neck, shoulders, arms, and hands are prevalent worldwide (1–4) and are common causes of absence from work and disability (5). MSD impose a large economic burden on society (1, 3, 4, 6–11).
Options for screening, surveillance, and diagnosis of upper-extremity MSD are limited and most depend on symptoms, which are by definition self-reported, or on functional ability. A need for improved diagnostic and screening methods, especially objective techniques, has been identified (12, 13). According to the US Institute of Medicine (IOM), a biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (14). In addition to improving diagnosis and screening methods (15), valid and reliable MSD biomarkers could aid in evaluating treatments and workplace interventions. Finally, such biomarkers could aid in elucidating MSD pathomechanisms.
The US National Research Council (NRC) has distinguished between three classes of biomarkers: exposure, effect (disease), and susceptibility (16). A biomarker of effect may be defined as “any change that is qualitatively or quantitatively predictive of health impairment or potential impairment…” (16). In this review, we focus on biomarkers of effect, which – through their window on pathophysiological disease processes – could potentially be utilized to stage the severity of a MSD.
Previous biomarker reviews
The potential for MSD biomarkers to detect subclinical disease and monitor MSD severity was discussed as early as 1992 by Mastin and colleagues (17). They recommended the use of animal models for biomarker research with subsequent validation in human populations. Since then, only one review focusing exclusively on biomarkers of MSD in humans has been published (18). Saxton (2000) (18) recommended longitudinal studies in which initially asymptomatic persons were enrolled, and then inflammatory substances and proteins specific to skeletal muscle be examined in relation to low force, highly repetitive tasks. In the intervening years, literature on biochemical markers of MSD has grown, although very few longitudinal studies have been performed. Recent reviews have highlighted biochemical alterations associated with the pathogenesis of particular MSD in humans or animal models (19–22). However, a systemic summary of the current state of MSD biomarker research in humans is needed. Therefore, we sought to conduct a comprehensive assessment of the current state of neck and upper-extremity MSD biochemical biomarker research, using two research questions: (i) Are there biochemical markers associated with neck and upper-extremity MSD? (ii) Are there biochemical markers associated with the severity of neck and upper-extremity MSD?
Methods
Review team and overview
Our review team consisted of ten researchers with expertise in epidemiologic, intervention, and experimental studies within the field of work-related MSD research. In a first step, we formulated research questions and defined principal concepts of the review. Then we developed a search strategy and search terms and conducted a search using PubMed and Scopus databases, with results pooled with articles identified from our own records. Thereafter, papers were screened based on pre-defined criteria (supplementary tables S1–S3, www.sjweh.fi/data_repository.php) using a two-step procedure of primary (title and abstract) and secondary (quality) screens. Summary tables were created from papers of sufficient quality, and evidence was synthesized with respect to the two research questions.
A consensus process was used throughout. The review team agreed on the definitions for neck and upper-extremity MSD and biochemical markers, operationalized severity, and developed specific inclusion/exclusion criteria.
Neck and upper-extremity MSD were defined as musculoskeletal symptoms or clinical diagnoses in the neck, shoulder, arm, wrist, or hand. These included both specific and non-specific conditions related to tendons, muscles, ligaments, blood vessels or nerves (23). We focused on MSD that occur in a work-related context (24).
Biochemical marker was defined as a substance obtained through biological fluids or tissues that may be evaluated as an indicator of normal biological or pathogenic processes. Such markers could be derived from serum, blood, urine, synovial fluid, saliva, muscle interstitium or through tissue biopsy samples. Genetic markers such as RNA, DNA or their constituents were considered as beyond the scope of this review.
Severity was operationalized as encompassing cross-sectional and longitudinal differences in symptom severity, or differences in extent of disorder in same anatomical segment (eg, size of rotator cuff tear).
Inclusion criteria
This review was limited to studies on humans and non-traumatic MSD of the neck and upper extremities. As categorized by Boocock et al (23), potential biomarkers were examined for the following specific MSD: flexor-extensor peritendinitis or tenosynovitis of forearm-wrist region, de Quervain’s disease (tenosynovitis of sheath/tunnel surrounding tendons controlling thumb movement), carpal or radial tunnel syndrome, Guyon (ulnar) canal syndrome, cubital tunnel syndrome (ulnar nerve entrapment at the elbow), epicondylitis (lateral and medial), secondary Raynaud’s phenomenon associated with vibration exposure, thoracic outlet syndrome, rotator cuff syndrome/shoulder tendonitis, and shoulder capsulitis. Non-specific diffuse forearm pain and other non-specific localized neck and upper-extremity MSD were included, as were upper-extremity pain in specific regions, including “shoulder pain”, “neck pain”, “neck/shoulder pain”, “elbow pain”, “arm pain”, and “hand pain”. Other specific MSD included are listed in Boocock et al (table 7) (23), although cervico-brachial fibromyalgia status post-whiplash and joint-related conditions were excluded. Data from immunohistochemistry studies in which antibody assays were used [similar to an enzyme-linked immunosorbent assay (ELISA)] were included, and if an in-vitro study using cells from muscle or tendon biopsies included data from unstimulated cells, then that latter data were included. We included articles that fulfilled our inclusion criteria, even if some parts of the study were consistent with the exclusion criteria; however, only results in compliance with our criteria were taken into account.
Exclusion criteria are summarized in supplementary table S1 (www.sjweh.fi/data_repository.php).
Literature search
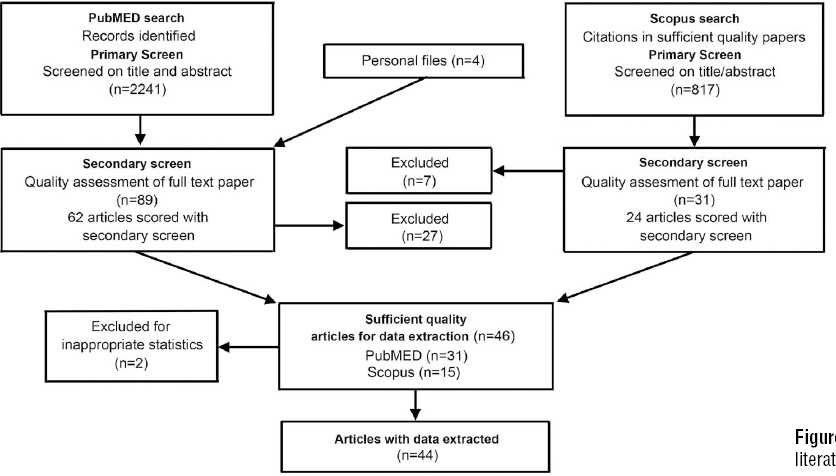
The search was first conducted in PubMed and combined with relevant articles identified from personal files. A second search for papers that cited the sufficient-quality articles was conducted using Scopus. See supplementary table S2 (www.sjweh.fi/data_repository.php) for an overview of the search strategy; figure 1 illustrates the search strategy and selection procedure. PubMed search terms included both MESH terms and keywords selected for two categories: upper-extremity MSD and biomarkers. We only included search terms which contributed more than two papers to the search. This elimination of papers did not result in having a lack of reviewable studies in any of the diagnostic categories listed in Boocock et al (23).
We identified 15 relevant articles and used these to refine the search and test its sensitivity. The search was restricted to peer-reviewed studies on humans, adults (>18 years of age), and English language text published within the last 25 years (June 4, 1988–June 4, 2013). See appendix 1 (www.sjweh.fi/data_repository.php) for the search string. The final PubMed search resulted in 2241 articles; four additional eligible papers were added from personal files (for a total of 2245 examined). A primary and secondary screening process was implemented (see below and supplementary tables S1–S3). After identifying the sufficient-quality articles, a second search was conducted in Scopus to identify relevant papers citing these articles. The main reason for the additional search in Scopus was that recently published articles in PubMed may not yet have been assigned MESH terms. Also, as our search strategy in PubMed may have missed some studies of relevance from other databases, the Scopus search was intended to reduce such potential bias. Pilot testing of forms and evaluation criteria was conducted at each stage of the review process (primary screen, secondary screen, data extraction) to assure clarity in review instruments and to limit bias among the reviewers.
Primary screen-selection of relevant articles
After importing PubMed records into systematic review software (EPPI-reviewer4 v4.3.4, EPPI-Centre at the Social Science Research Unit at the Institute of Education, University of London, UK), two independent reviewers conducted a primary screen assessing each title and abstract for eligibility based on the criteria in supplementary table S1 (www.sjweh.fi/data_repository.php). The fulltext article was read if necessary. A “yes” answer on any of the questions resulted in exclusion of the article. Results were compared between reviewers, and a consensus agreement was reached (see below). The same procedure was repeated for articles from personal files and additional articles identified using Scopus.
Secondary screen-quality assessment & data extraction
All articles passing the primary screen were scored for quality. The articles were randomly allocated to five different clusters, and each of five reviewers was assigned randomly to two clusters. The quality screen procedure was as follows: each article was assessed by two independent reviewers, scores were compared between reviewers, and a consensus agreement was reached after discussing disagreements. If an agreement could not be reached, a third person from the review team was involved. The questions used for the quality assessment are listed in supplementary table S3 (www.sjweh.fi/data_repository.php). These questions were derived from reporting guidelines for research studies and checklists assessing study quality in health-related research (25–29). The scoring system contained 17 items, where each item could be scored as either “yes” (1 point), “unknown or not applicable” (0), or “no” (0). Scores were summed for each paper (ranging from 0–17). To determine a criterion for article data extraction, a sample of five papers were scored by two reviewers. Based on our judgment of quality, we determined that articles scoring ≥65% of the maximum (11/17) should be included for data extraction. These articles were labeled as “sufficient quality”. Regardless of quality, articles scoring “no” on question 15 were excluded from data extraction. These included papers with less appropriate statistical analysis methods, such as multiple comparisons without adjustment, and modeling without accounting for repeated measures. See appendix 2 for data extraction items (www.sjweh.fi/data_repository.php).
Research synthesis
Next, we evaluated the number of sufficient-quality articles identifying a particular biomarker or class of biomarkers in its association with MSD in conjunction with a best evidence synthesis approach (30). Considering the heterogeneity of biomarkers and MSD studied, it was not possible to conduct a meta-analysis.
We did not expect a universal biomarker or biomarkers for the variety of MSD examined. Results for biomarkers were categorized by MSD in the tables, and summarized by physiological process in the text (eg, collagen degradation and repair). This dichotomy in presentation was chosen as we anticipate that our target audience is more familiar with MSD diagnoses. It should be noted that MSD diagnoses do not necessarily reflect underlying pathophysiology, and common physiological processes may underlie multiple MSD.
Physiological processes for which there were five or more relevant papers are presented. A biomarker was considered to belong to a category based on known physiological processes or, if several categories were appropriate, on the processes presented in the original article. In experiments with biomarker values across several time points, we considered any statistical comparison between cases and controls (or tests of association with MSD severity) cases) to be relevant.
An association between the biomarker and MSD in three or more sufficient-quality studies (and at most one sufficient-quality study with a null finding) was regarded as evidence that a substance could serve as a MSD biomarker. On a per biomarker basis, the smallest percentage of papers that demonstrated an association was 75%.
Results
Of the 2241 articles identified in the primary PubMed review (figure 1), plus the 4 papers added from authors’ personal files, 89 met the inclusion criteria for secondary (quality) screening. We excluded 27 papers upon viewing the entire article and 31 of the remaining 62 articles met the sufficient-quality criteria score of ≥11. Next, we searched Scopus for articles that cited these 31 papers. Of 817 citations initially found in Scopus, 31 were determined to be non-duplicates and of relevance through a title and abstract review; 7 were eliminated at the secondary screening stage upon viewing the entire article. Hence, 24 articles were scored with the secondary screen. Of these, 15 were scored at ≥11, adding to the 31 similarly scored articles found in the initial PubMed search. These 46 studies were regarded as being of sufficient quality to merit data extraction, except for 2 that used less appropriate statistical analysis methods. Hence, data was extracted from 44 articles.
Secondary screen-quality assessment overview
Of all of the studies meeting the criteria for quality scoring (N=86; 62 from PubMed and 24 from Scopus), the majority had clearly defined aims, biomarkers, MSD, and results (supplementary table S4, www.sjweh.fi/data_repository.php). Most studies had a cross-sectional design and utilized convenience sampling of patients, for both cases and referents. The response rate was explicitly stated or easily inferred in only 11 studies. Of these, the response rate to an invitation to participate was >65% in 9 (31–39). Less than half of the 86 studies controlled for confounding factors through restriction of subjects to a particular age or gender, or through adjustment in the analysis. Thirteen studies (15%) explicitly stated that biomarker analyses were blinded to case conditions, and 27 (31%) used less appropriate statistical analysis methods.
Data extraction from sufficient-quality studies
The majority of included studies examined neck/shoulder disorders and symptoms, with shoulder disorders, Dupuytren’s contracture, and hand-arm vibration syndrome (HAVS) also well represented (supplementary table S5, www.sjweh.fi/data_repository.php). Please reference appendix 3 for definitions and descriptions of abbreviations and biomarker analyses presented in the tables. Four studies examined upper-extremity MSD or symptoms without further specifying a particular body region (38, 40–42). Referent groups ranged from healthy controls to patient groups with conditions presumed to be sufficiently unrelated to the biomarker(s) under investigation. Only one was a longitudinal (non-treatment) study (37).
Many studies were conducted in older populations with at least one analysis group >50 years of age (33–35, 43–60). The range of symptom duration varied substantially within and across studies. Of the 15 studies that listed an average or range of symptom duration, nearly half involved participants with MSD symptoms of >70 months (59, 61–65). Three reported extremely wide-ranging MSD durations (49, 50, 66) (66: 7–120 months; 49: 7–360 months; 50: 9–288 months). Five studied patients with an average symptom duration of <1 year (33, 40, 41, 54, 56).
Are there biochemical biomarkers associated with neck and upper-extremity MSD?
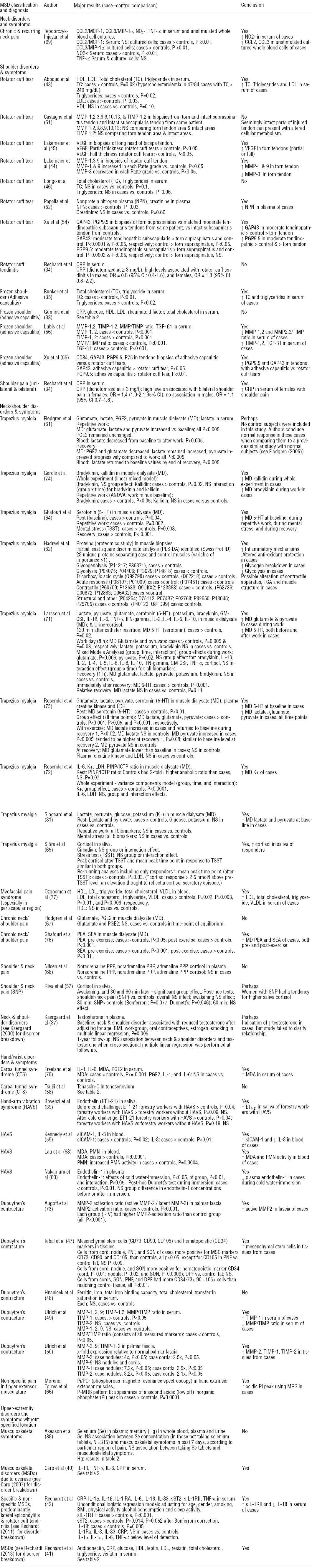
The majority of studies demonstrated an association between at least one biomarker and the MSD examined (table 1). Only 8 of the 44 studies reported insignificant findings throughout (33, 38, 46, 48, 51, 58, 67, 68). Descriptions are provided below for the categories of biomarkers examined in >5 studies. See appendix 3 for definitions of abbreviations and biomarker analyses (www.sjweh.fi/data_repository.php).
Inflammatory
Fourteen studies examined biochemical mediators and markers related to inflammatory processes (33, 34, 40–42, 59, 61–63, 67, 69–72). Seven found significant associations between inflammatory markers and MSD (34, 40, 42, 59, 62, 63, 69), while seven did not (33, 41, 61, 67, 70–72). Where associations were found, all had increased levels of inflammatory substances in MSD cases or with increased MSD severity, except for two studies in which reduced levels of IL-8 and IL-18 were observed in cases (41, 59).
A great breadth of inflammatory substances was examined. Six studies examining inflammatory indices in serum or blood observed increases: (i) serum macrophage chemotactic factor-1 (CCL2/MCP-1) and macrophage inflammatory protein-1 alpha (CCL3/MIP-1α) in cultured whole blood cells with chronic neck pain (69); (ii) serum C-reactive protein (CRP) with bilateral shoulder pain in females (34); (iii) serum CRP, IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) with proximal and distal upper-extremity MSD of <3 months duration (40); (iv) serum sIL-1RII with specific and non-specific upper-extremity MSD (42); (v) serum soluble intercellular adhesion molecule-1 (sICAM-1) with HAVS (59); and (vi) blood polymorphonuclear cells (PMN) cell activity with HAVS (63). Associations between serum inflammatory cytokines, mediators and biomarkers were not consistent across reviewed studies. No association was observed between serum CRP and rotator cuff tendinitis, adhesive capsulitis, or a mix of MSD of <1 month duration (33, 34, 41). No association was found between serum TNF-α levels (an acute inflammatory cytokine) and chronic neck pain (69), between serum IL-1 and IL-6 levels (a pleiotropic cytokine and myokine) and late stage carpal tunnel syndrome (70), or between four acute phase cytokines (IL-1α, IL-1β, IL6, TNF-α) and a mix of MSD as each were below detectable levels (41). Results were less mixed when using local sampling methods, eg, assays of analytes from muscle extracellular fluid obtained using microdialysis. No association was observed between several inflammation-related cytokines in the muscle interstitium and trapezius myalgia (71, 72). No association was observed for muscle interstitial prostaglandin E2 (PGE2) and trapezius myalgia and chronic muscle pain in the neck/shoulder (61, 67), although it should be noted that PGE2’s role is not as a principal driver of inflammation but more as a mediator involved in immune responses. In muscle biopsies, Hadrevi et al (62) found increased heat shock 70 kDa (HSP 70) protein and alpha-1-antitrypsin, proteins involved in the inflammatory response.
In summary, results were largely inconsistent for the inflammatory substances examined in more than one study. The exception was a null finding for PGE2, which was observed in trapezius myalgia, neck/shoulder pain, and carpal tunnel syndrome.
Collagen degradation and repair
Seven studies examined biomarkers of collagen degradation and repair, a category that included matrix metalloproteinases (MMP, collagen degradation markers), tissue inhibitors of matrix metalloproteinases (TIMP), cross-linked carboxyterminal telopeptide of type I collagen (ICTP, a collagen degradation marker), and procollagen I intact N-terminal propeptide (PINP, a collagen type I synthesis marker) (44, 49–51, 56, 72, 73) (table 1). All but one study (51) found a significant association between at least one biomarker of collagen degradation and repair and the MSD under investigation.
Three studies found greater concentrations of collagen degradation markers in MSD cases than controls, specifically increased MMP in synovial fluid and biopsies from subjects with torn rotator cuff tendons and palmar fascia biopsies from subjects with Dupuytren’s contracture (44, 50, 73). A study of adhesive capsulitis found reduced MMP in patients (56). Two studies saw no association with this category of potential biomarkers (49, 51).
Three studies found increased TIMP (most consistently, TIMP-1) in serum from subjects with adhesive capsulitis, and in serum or palmar fascia from subjects with Dupuytren’s contracture (49, 50, 56). Two of these showed a decreased MMP/TIMP ratio in serum of subjects with fibroproliferative MSD (49, 56). A study where the collagen synthesis-to-degradation ratio (PINP/ICTP) was examined in muscle interstitial dialysate found no association in subjects with and without trapezius myalgia (72). The one study that did not find a significant association with collagen-repair markers used adjacent tendon regions from subjects with torn supraspinatus tendons as internal control tissues, and found increased TIMP and MMP in both the torn and seemingly intact adjacent tendon regions (51). These authors postulated that tendon areas near injured tendons can present with altered cellular metabolism.
In summary, the most consistent result was increased TIMP-1 (a collagen-repair marker) in serum or tissue from subjects with Dupuytren’s contracture and adhesive capsulitis. Additionally, collagen turnover (MMP/TIMP level) was decreased in these subjects.
Muscle metabolites/neurotransmitters
Eleven studies examined muscle metabolites/neurotransmitters using local sampling methods (31, 61, 62, 64, 66, 67, 71, 72, 74–76). Eight of these found at least one significant association between a biomarker and the MSD (31, 62, 66, 71, 72, 74–76), while three found no association (61, 67, 76). Except in one study (62), the concentration of the biomarker was greater among cases than controls. In one study (62), an increase was noted for 11 possible biomarkers and a decrease for 16. In all but one study (66), biomarkers were measured in the trapezius muscle interstitium or via muscle biopsy. In most of the muscle microdialysate experiments, a rest (baseline) period was followed by a repetitive physical or mental work challenge and then a subsequent recovery period.
Several studies reported higher levels among cases than controls of muscle metabolites that play a role in regulating pain. This was observed with lactate and pyruvate at baseline (31) and with glutamate (75), kallidin (74), lactate (75), and potassium (K+) (75) across an experiment. Three studies reported elevated bradykinin (74), glutamate (71), and pyruvate (71) among cases versus controls during repetitive work. Another study (61) also observed increased glutamate, lactate, and pyruvate in cases during work versus baseline levels. In contrast, Sjogaard et al (31) observed no significant changes in these muscle metabolites with work or mental stress, despite increased lactate and pyruvate among cases at baseline compared to controls (31). With regard to the recovery period, two studies reported a rapid return of these metabolites to baseline levels (71, 75), while one (61) observed maintenance of work-induced increases in lactate and progressively increasing pyruvate levels during the recovery period. In muscle biopsies from subjects with trapezius myalgia, one study showed increased enzymes in glycogenolysis and the initial steps of glycolysis but a decrease of enzymes in the final steps of glycolysis (62).
In the four studies that examined glutamate, one observed an elevated level in cases during the entire experiment (75), while another found an elevation among cases versus controls during work but not during recovery (71). These results differ considerably from those of Flodgren et al (67) who observed no differences in biomarker concentration between cases and controls at rest. A repeat of this study (61) showed an elevation in cases of glutamate during repetitive work versus baseline (there was no control group), although they concluded that this was a normal increase due to muscle metabolism.
Of the four studies investigating lactate, one observed an elevation in cases versus controls during the entire experiment (75) while another (71) found no overall difference between cases and controls. Sjogaard et al (31) observed a comparative elevation in lactate during baseline, but not in response to repetitive physical or mental work, or during recovery. In contrast, Flodgren and colleagues (61) found increased lactate during repetitive work.
Of the four studies that examined pyruvate, one observed an elevation during the entire experiment (75), one observed an elevation at baseline only, but not in response to work or during recovery (31), and one observed an increase in response to work, but not during recovery (71). One study (61) found increased pyruvate during repetitive work.
Three studies examined 5-HT in muscle dialysates, a monoamine neurotransmitter known to sensitize nociceptors, and found increases in cases with trapezius myalgia and chronic neck/shoulder pain at baseline versus controls (64, 71, 75). One study (64) found increased 5-HT levels in cases during both physical and mental work, while two found increased 5-HT in cases during recovery (64, 71). Another study examining two other muscle metabolites that regulate pain responses, N-palmitoylethanolamine (PEA) and stearoylethanolamide (SEA), found a significant association between levels of these analytes in muscle dialysate in subjects with chronic neck pain, compared to controls (76).
Lastly, one study examined local muscle changes in the hand using phosphorous magnetic resonance spectroscopy. Moreno-Torres et al (66) observed an increased peak of acidic inorganic phosphate (Pi) in hand muscles in subjects with non-specific pain in finger musculature. This factor is thought to induce fatigue by interfering with biochemical steps in the cross-bridge cycle.
In summary, results with respect to muscle metabolites/neurotransmitters were mixed with the exception of Pi, PEA and SEA, which were examined in only one study each, and 5-HT in which increases were generally observed in trapezius myalgia cases.
Lipids/insulin resistance
Seven studies investigated lipids and cholesterol using systemic sampling methods, ie, blood samples (33, 35, 41, 43, 46, 48, 77). Four observed statistically significant associations with MSD (35, 41, 43, 77); three did not (33, 46, 48). Total cholesterol (TC) was examined in all seven studies. Two studies investigated rotator cuff tears (43, 46), showing increased (43) or no difference in TC (46) among cases versus controls. Two studies investigated frozen shoulder (adhesive capsulitis), also with divergent results. One study found greater levels of TC in cases with frozen shoulder (35), whereas one did not (33). No association was found between TC and Dupuytren’s contracture (48). Ozgocmen et al (77) found an association between increased TC in subjects with myofascial pain versus controls. When investigated, no correlation between TC and severity level was found (33, 41).
The association between serum triglycerides and MSD was studied in five papers. Of those studies investigating rotator cuff tears (43, 46), one showed increased triglycerides in cases (43) and one found no difference between cases and controls (46). Increased triglycerides were observed in adhesive capsulitis (35) and myofascial pain (77), and among MSD cases reporting greater pain intensity (41).
Four studies investigated low- and/or high-density lipoprotein (LDL and/or HDL) in cases with MSD. In cases with rotator cuff tears, an increased LDL was shown (43) and, in adhesive capsulitis, both LDL and HDL were increased (33). In a myofascial pain study, LDL was increased but HDL was not (77). A decreased concentration of HDL was found among MSD cases reporting greater pain intensity (41).
In summary, results with respect to lipids including cholesterol were mixed, with the exception of triglycerides where increased levels were generally observed among cases.
Are there biochemical biomarkers associated with the severity of upper-extremity MSD?
As shown in table 2, five studies demonstrated a relationship between MSD severity and biomarker concentration (40, 41, 49, 50, 76). Six studies investigated biomarker concentration in relation to a severity score or disease stage determined during a physical examination (33, 40, 45, 49, 50, 73). Increased collagen-repair marker, TIMP-1 (49, 50), and a decreased MMP/TIMP ratio (49) were observed at an earlier (more active) Dupuytren’s contracture stage. Increased MMP-2, a collagen degradation marker, was found in an earlier stage of Dupuytren’s contracture in one study (50), although not in another (73). Carp et al (40) found positive correlations between inflammatory markers IL-1β, IL-6, and TNF-α and MSD severity in a group of early stage (mean 1.7 months) patients.
Table 2
Are there biochemical biomarkers associated with the severity of neck and upper-extremity musculoskeletal disorders (MSD)?
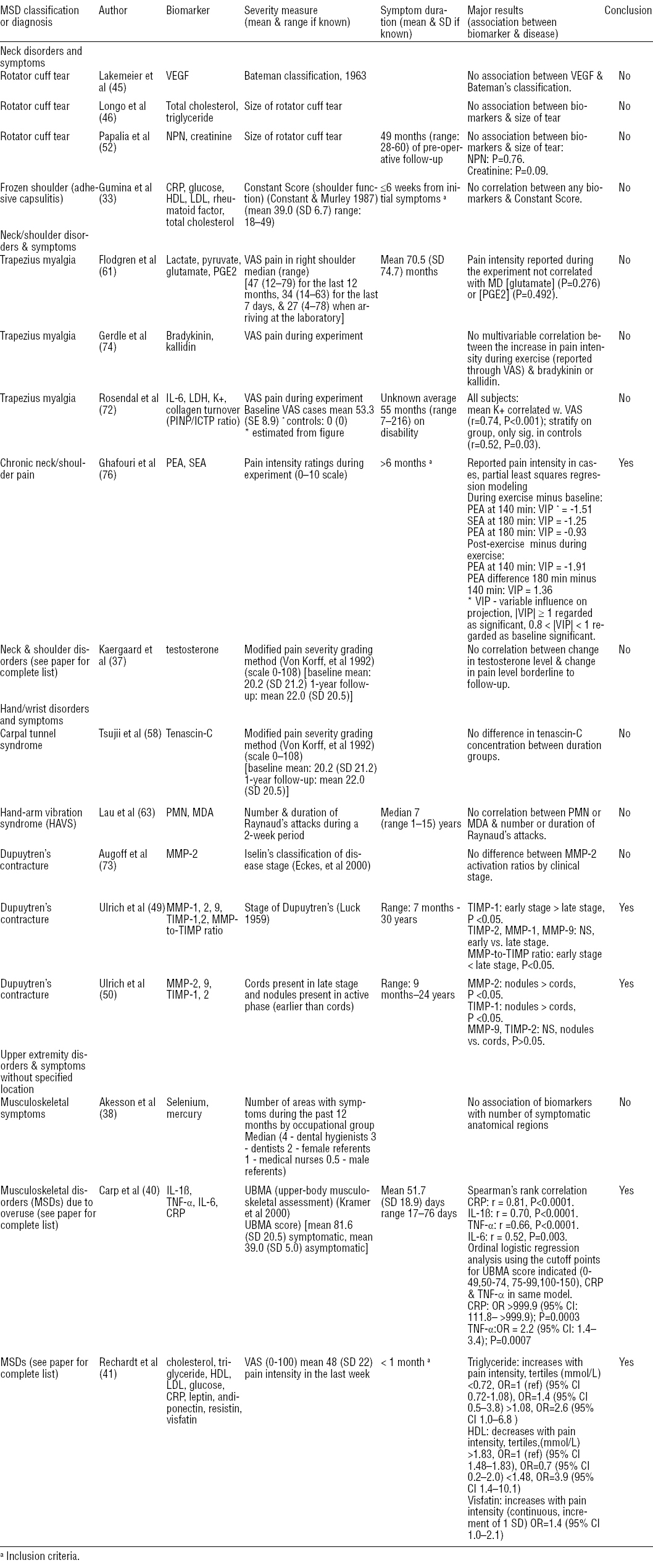
Six studies solicited pain ratings from participants (37, 41, 61, 72, 74, 76). Ghafouri et al (76) found increased concentrations of PEA and SEA when patients reported smaller differences in pain between exercise and baseline, or between exercise and recovery periods. In Rechardt et al’s study of subjects with MSD of >1 month (41), triglyceride and visfatin were increased and HDL decreased among those reporting greater pain.
In summary, only a few studies found associations between biomarkers and severity of upper-extremity MSD. Results with respect to inflammatory markers were mixed. While Carp et al (40) observed increased inflammatory marker concentrations with increased MSD severity in a mix of MSD, Ghafouri et al (76) found increased inflammatory marker concentrations in chronic neck/shoulder pain patients reporting a lower increase in pain in response to repetitive low force work. Lastly, increased collagen-repair markers were found in earlier stages of Dupuytren’s contracture (50).
Discussion
We conducted a comprehensive systematic review to summarize the current state of biochemical marker research in neck and upper-extremity MSD. We used a critical approach to synthesize results for the research questions: (i) are there biochemical markers associated with neck and upper-extremity MSD? and (ii) are there biochemical markers associated with the severity of neck and upper-extremity MSD? We found associations between biochemical biomarkers and neck and upper-extremity MSD in the sufficient-quality studies. A wide range of biomarkers were examined. Nonetheless, with several exceptions listed below, there was little consistency of results. However, we were able to identify a few commonalities.
An increased collagen-repair marker, TIMP-1, and decreased collagen turnover (MMP/TIMP ratio) was observed in serum among cases with Dupuytren’s contracture (49) and early stage frozen shoulder (56). TIMP-1 was also increased in tissue from Dupuytren’s contracture cases relative to normal palmar fascia (50). Elevated serotonin (5-HT) was found among muscle interstitium in trapezius myalgia cases (64, 71, 75, 76). Increased triglycerides were seen in serum of subjects with rotator cuff tears (43), frozen shoulder (35), myofascial pain (77), and subjects reporting greater pain intensity with mostly tendon-related MSD (41). In contrast to the first research question, associations between biomarkers and severity of neck and upper-extremity MSD were observed in only a few studies. Greater collagen degradation and repair is likely occurring in earlier stages of Dupuytren’s contracture (49, 50).
Both Dupuytren’s contracture and frozen shoulder are fibroproliferative disorders (78). Biomarker papers examined in this review suggest that an imbalance in extracellular matrix remodeling may be occurring, similar to other fibroproliferative conditions (79). MMP, which degrade collagen, are active in wound repair, whereas TIMP are inhibitors of MMP. An increased concentration of TIMP may result in fibrosis. Two studies demonstrated increased TIMP-1 in serum of subjects with fibroproliferative disorders and an inverse association of the MMP/TIMP ratio in MSD cases (49, 56), consistent with the above interpretation.
In a previous review of microdialysis studies in human myalgia and tendon-related conditions (20) and in a review of microdialysis studies specifically focused on chronic muscle pain conditions (22), the authors concluded that 5-HT may potentially be a biomarker of chronic myalgia; we concur with their conclusions. They note that 5-HT can activate nociceptors which transmit pain signals to the central nervous system, and that it may have an algesic or hyperalgesia effect depending on the particular cell and receptor targeted (20). Potential involvement of this mechanism in trapezius myalgia should be further examined in longitudinal studies.
A number of studies found associations between triglyceride concentration and MSD. However, there is insufficient evidence to suggest that triglycerides are a necessary condition for MSD occurrence. Increased serum triglycerides are often found in obesity, metabolic syndrome, and diabetes. Thus, it is likely that the observed increases in triglycerides with MSD are due to comorbidity of these conditions.
Nonetheless, several mechanisms have been hypothesized by which hyperlipidemia may increase tendinopathy risk. First, lipids (xanthoma) may be deposited on tendons which may alter their biomechanical properties (43). Xanthoma may also initiate a low-grade inflammation in tendons, initiating chronic tendon degeneration (80, 81). Lastly, lipids may alter the extracellular matrix and reduce circulation, both impairing tissue healing (43). These possible lipid effects on tendons combined with metabolic and biomechanical alterations due to tendon repetitive loading (82, 83) may increase MSD risk.
Methodological limitations of the articles in the review
Selection bias
It was only possible to ascertain the response rate to an invitation to participate in 9/44 sufficient-quality studies. In many articles, it was stated that patients were enrolled consecutively, but the control group response rate was not stated. Without response rates, it is not possible to adequately assess selection bias, ie, whether cases and controls fairly represent the underlying population, or to what extent cases and controls may be comparable. Future studies should delineate the response rates for both cases and controls.
In addition, there is potential for bias due to selection of referents. For our first research question, we evaluated studies that compared subjects with the MSD or symptom(s) of interest to one or more referent groups. The referent group sometimes consisted of asymptomatic participants. However, several studies utilized convenient patient populations as controls (supplementary table S5, www.sjweh.fi/data_repository.php). These may not necessarily be appropriate controls for the biomarkers under investigation. Some studies of shoulder disorders used referents with shoulder disorders presumably affecting different anatomical systems than the condition under study. For instance, in Lakemeier et al’s studies of rotator cuff tears (44, 45), patients with traumatic humeral head fractures were utilized as controls. However, the biomarkers studied could be present in both cases and controls. Vascular endothelial growth factor (VEGF) promotes both angiogenesis and bone formation (84), and genes encoding MMP were found to be up-regulated in fracture repair (85). Concentrations of the biomarkers examined were greater among cases than controls in Lakemeier et al’s studies (44, 45). Nonetheless, when individual studies each examine a particular biomarker for a specified disease, yet select unhealthy controls with differing disorders, mixed results may occur. Selection of controls from the same hospital as cases can be advantageous (in that confounding factors are more apt to have similar distributions within groups); nevertheless, disease processes among unhealthy controls could potentially affect the presence or absence of the biomarkers being studied. Likewise, collecting tissues during surgery from nearby seemingly “intact” tissues to use as internal negative controls may lead to confusing results as both may have altered cellular metabolism, as was found in the Castagna et al study in which MMP and TIMP were elevated in both torn and adjacent “intact” tendon regions (51).
Spectrum effect (variation of biomarkers across subgroups)
The term spectrum effect has been used to describe heterogeneous diagnostic test results in different patient subgroups (86). We apply it here to refer to the potentially heterogeneous biomarker associations in populations according to symptom severity or duration, age, gender, and the like. Many of the sufficient-quality studies in this review have been conducted among older patients at the time of surgery (44, 45, 47, 48, 50, 54, 58). Comparatively fewer authors have researched MSD biomarkers in younger subjects with incipient disorders (40, 41). Animal models would suggest that different biomarkers are present at different MSD stages and may differ by age (87–89). Various theories of MSD pathophysiology also suggest that differing biochemical biomarkers would occur at different MSD stages (21, 82, 90). The literature reviewed in the present paper does not allow for any empirical evaluation of the above assertion because there were an insufficient number of studies that detailed symptom duration. We recommend that the duration and/or symptom range of cases in any given biochemical biomarker study be reported. We further suggest that the duration and/or symptom range be restricted or results be analyzed according to duration and/or symptom indicator. Certainly studies enrolling patients with less severe disease than surgical cases, and studies with MSD cases <50 years of age are warranted for future research.
Confounding
Approximately half of the sufficient-quality studies reviewed controlled for potential confounders, eg, gender or age, either through restriction of study subjects or through adjustment in the analysis. It is possible that there was a randomization of potential confounders among the analysis groups in the studies where confounding was not controlled for, which would not invalidate the research. However, potential confounding factors in the relationship between biomarkers and MSD should at least be mentioned in future studies. More ideally, information on these factors should be collected from study subjects and controlled for, either through selection or in the analysis.
Comorbid conditions
The majority of biomarkers in this review are certainly not specific to the MSD studied. Thus, careful consideration of subject comorbidities is warranted in order to avoid spurious associations. For instance, one inflammatory marker, IL-1β, may be up-regulated in rheumatoid arthritis, gout, osteoarthritis, type 2 diabetes, multiple myeloma, and post-myocardial infarction heart failure (91). In another example, CRP is a sensitive biomarker of inflammation and has been used to identify in individuals with unstable angina pectoris (92), and underlies coronary artery disease and risk of future stroke (93, 94). We recommend careful attention to comorbidities in future research since the specificity of potential biomarkers needs to improve to be useful in clinical practice.
Heterogeneity – other considerations
The collection method (eg, blood, microdialysis of muscle interstitial fluid, tissue biopsy), and detection method used to assay an analyte (eg, single plex versus multiplex ELISA, radioimmunoassay (RIA), immunohistochemistry) may account for some varying results. A recent study compared single-plex versus multiplex ELISA kits for detection of inflammatory cytokines and chemokines in plasma (95). Compared to single-plex ELISA, the multiplex ELISA system proportionally and significantly overestimated IL-1β and interferon (IFN) gamma, but underestimated IL-6. The authors noted that such differences may distort epidemiologic relationships if data from both methods are merged (95). Tubes selected to collect blood samples can also affect results based on the analyte being assayed or planned detection system (eg, ELISA versus Western Blot analysis) as different anti-coagulants in the tubes can be problematic with some detection systems (96). Other considerations for best sample collection, storage and implementation of internal controls for clinical trial studies are reviewed by de Jager et al (96).
Additional gaps in the literature
The majority of the sufficient-quality studies focused on neck/shoulder or shoulder disorders, with nine conducting research on trapezius myalgia, and seven researching rotator cuff tears. There is a paucity of biomarker studies of arm and hand MSD. Although Dupuytren’s contracture studies were represented here, we found no sufficient-quality studies researching forearm-wrist tendinitis, epicondylitis, or de Quervain’s tenosynovitis. Biomarker studies of MSD in the lower extremity could inform upper-extremity MSD research. For instance, there is a rather sizable body of literature on Achilles tendinopathy [eg, see (97–99)] that may be applicable to upper-extremity tendinopathies. Such articles were beyond the scope of the present review.
For most MSD, pathophysiological mechanisms are not well understood. Many researchers chose biomarkers based on theoretical models of MSD pathophysiology. However, some were less grounded in previous work. To some extent it appears plausible that some biochemical biomarkers were selected primarily on the basis of assay with easy availability, and/or low cost. While exploratory studies are necessary, a key concept in improving biomarker discovery would be to choose potential markers with reference to MSD animal models and/or proposed pathomechanisms. An alternative approach to individual testing of a few potential biomarkers would be to use different pattern analysis methods on larger sets of predefined potential markers (selected on either a theoretical or empirical basis). The biomarker could be a specific pattern of individual markers rather than a single substance (100).
Strengths and limitations of the review
This review makes a contribution by systematically synthesizing the literature on biomarkers and MSD. This is the first comprehensive review of biochemical biomarkers in human neck and upper-extremity MSD since 2000 (18). Additionally, it is the only such review that we are aware of to address the question of MSD severity. Since different MSD affect a variety of anatomical systems with differing localization of symptoms, the pathophysiological mechanisms within each anatomical system may differ from each other and thus express separate, relevant biomarkers. However, MSD may also affect multiple anatomical regions (101–103), thus yielding either expression of systemic markers or a pattern of more specific markers related to a specific anatomical segment. We attempted to analyze the results on an MSD basis (separate analysis for each MSD). However, there was little consistency of biomarkers studied within one particular MSD. Hence, we also looked more broadly at classes of biomarkers or at individual biomarkers for multiple upper-extremity MSD.
As with all reviews, publication bias (in which studies with statistically significant results are more apt to be published than studies with null findings) may certainly be a factor in this review. Although many studies with null findings were reviewed, it is possible that even more such studies have been carried out and were not published. Another concern is selective reporting of positive findings. Several articles in this review have yielded mixed results for the biomarkers studied, although it is possible that some authors only reported their statistically significant findings. Since rheumatic or widespread pain conditions were excluded from this review, it is conceivable that a bias was introduced which may have selected out more severe MSD that affect numerous anatomic sites concurrently – perhaps those that triggered an autoimmune response affecting widespread anatomical sites.
Recommendations
Our review shows that biochemical biomarker research for neck and upper-extremity MSD is at a nascent stage. Briefly, these are our recommendations for future biochemical biomarker research:
-
Report the response rate for all analysis groups (nominally, for cases and referents).
-
Use healthy control subjects where possible.
-
Restrict subjects by duration and/or symptom range, or adjust for this in analyses.
-
Select biomarkers identified in MSD animal models and/or proposed pathomechanisms or use previously investigated biomarkers in order to verify findings in new populations.
-
Carefully consider and report potential confounders, gather information on these factors from study subjects, and potentially control for them through restriction or through adjustment in the analysis.
-
Consider analysis by disorder severity.
-
Prioritize biomarker studies that are longitudinal, include patients with early or intermediate stage disorders, and address patients with elbow, arm, or hand symptoms.
Concluding remarks
This comprehensive review used systematic methodology and demonstrated that although there are studies that show an association between biochemical biomarkers and upper-extremity MSD, the evidence is inconsistent. The most consistent studies suggest that 5-HT may be used as a biomarker for trapezius myalgia, and that TIMP and MMP/TIMP ratio may be used as biomarkers for selected fibroproliferative disorders. Further research is needed to confirm these conjectures. We have identified gaps in the literature and provided guidelines for future studies. Prospective high quality studies are needed to propel this field forward. Systematic biomarker selection and deliberate testing of biomarkers in different MSD is recommended. This testing should be done with regard to duration and severity of the MSD. Selection criteria and response rates of all analysis groups should be detailed, and confounding factors described. It would be advisable for researchers to select biomarkers in future studies based on MSD animal models or theories of MSD pathophysiology.