Carpal tunnel syndrome (CTS) is an impairment of the median nerve at the wrist with symptoms including numbness, tingling, and pain in the radial part of the hand (1). Recent reviews have concluded that the risk of CTS is increased in relation to repetitive work, particularly in combination with force exertion, while the influence of wrist postures is less well-documented (2–5). Occupational mechanical exposures may lead to increased pressure in the carpal tunnel and traction of the median nerve, which may initiate a series of changes such as ischemic microcirculation injury, edema, alterations in the blood-nerve barrier, thinning of myelin, altered ion channel dynamics and expression, and axonal degeneration (1, 6, 7).
Little is known about the time relation between entry into a job that entails high mechanical exposures to the wrist and the development and course of median nerve impairment. To the extent that an impairment of nerve function is reversible in initial phases, early identification may be important to prevent chronicity. Another perspective is that if a short induction period is followed by a reversible phase, surveillance of nerve conduction may be a responsive here-and-now assessment tool to guide workplace interventions to reach safe exposure intensities.
Two human studies have indicated a short induction period for CTS. One of the studies showed that surgery for CTS was most often performed within the first three seasons of work in a lamb slaughter and processing plant (8). In the other study, which followed newly hired pork processing employees, nerve conduction studies (NCS) showed signs of impaired median nerve conduction after an average of 64 work days (9). Recovery has been studied among assembly line workers with CTS, who tended to recover within two years, according to NCS after five months of reduced exposures (10).
Experimental animal studies have shown that 12 weeks of repetitive work with either high- or low-force exertion led to impaired median nerve conduction in rats (11, 12). In monkeys, a close temporal relationship was found between around 20 weeks of performing a moderately forceful, repetitive task and the development of abnormal NCS with subsequent recovery within weeks after exposure termination (13, 14). The authors found it unlikely that changes in humans would occur as rapidly (13).
The aim of this study was to evaluate median nerve function in relation to three weeks of hand-intensive seasonal work. We hypothesized that at end-season, median nerve conduction would be impaired and then recover within weeks.
Methods
Design and population
We conducted a prospective cohort study of 11 mink skinners from one mink skinning facility in Denmark, applying pre-, mid-, end-, and post-season NCS. Mink skinning is hand-intensive, seasonal work, which takes place during a few weeks each year, thus providing us with a natural experimental setting. We included mink skinners, who were Danish residents and could read and write Danish. Any previous physician-diagnosed CTS was an exclusion criterion. The Central Denmark Region Committees on Biomedical Research Ethics (record no. 1-10-72-263-14) and The Danish Data Protection Agency (record no. 1-16-02-84-14) approved the study. Written informed consent was obtained from all participants.
Exposure characteristics
Mink skinning was performed in day- and evening-shifts of 7.5 hours. For each participant, we obtained day-by-day accounts of the number of minks skinned from pay slips. Male and female minks had a weight of around four and two kilograms, respectively. From an adjacent table, the mink skinners grabbed one mink at a time using their left hand, and manually mounted it on a skinning machine. They pressed two buttons, one with each hand to activate the machine, and used a knife in their dominant hand to assist the last part of the skinning.
The same trained investigator performed full shift exposure measurements for the six day-shift workers; all measurements were dominant-sided. Postures and movements of the wrist were measured using SG75 twin axis goniometers (Biometrics Ltd., Cwmfelinfach, Gwent, UK) and recorded at a rate of 128 Hz using Mobi8-loggers (Twente Medical Systems International, Oldenzaal, The Netherlands) (15). To define 0° of flexion/extension and ulnar/radial deviation, the reference was a position with the forearm in pronation and the 3rd metacarpal bone aligned with the distal forearm’s axis (16). For each participant, the goniometer data was processed to yield the 10th, 50th, and 90th percentiles of the angular distribution of wrist flexion/extension (º; negative and positive values denote extension and flexion, respectively), % time with non-neutral wrist postures defined as flexion/extension >45º or ulnar/radial deviation >20º (17), the median velocity of wrist flexion/extension (º/s), the repetitiveness defined as the mean power frequency (Hz; for a strictly cyclic movement, this is equal to the inverse of the cycle time) (18), and % time with no movement defined as an angular velocity <1 °/s.
Bipolar surface electromyography (EMG) of the forearm extensors was used to measure force exertion (19, 20). We placed Ag/AgCl electrodes with an active diameter of 6 mm and a center-to-center distance of 20 mm (VS, Medicotest A/S, Ølstykke, Denmark) over the bellies of mm. extensor carpi radialis longus et brevis, located by palpation during voluntary contraction with pronated forearm, at a distance of one-third the forearm length from the elbow. Data was recorded at 1024 Hz using Mobi8-loggers (Twente Medical Systems International, Oldenzaal, The Netherlands). Root mean square values of the EMG amplitudes were calculated for periods of 1/8 seconds to describe the muscular activity. Data was normalized to the maximal voluntary EMG activity (MVE) as measured during the highest of three measurements of maximum handgrip force (Jamar dynamometer, Sammons Preston, Bolingbrook, IL, USA). MVE was measured with the participant seated, the elbow flexed 90º and the upper arm vertical (21). Data was processed to yield the 10th, 50th, and 90th percentiles of the amplitude distributions and % time with muscular rest defined as an activity <0.5 % MVE.
All measurement data was analyzed using EMINGO, a program for analyzing EMG, inclinometry, and goniometry, developed by the Department of Occupational and Environmental Medicine, Lund, Sweden. During the measurement day, the participants logged the start and end time of breaks, and exposure measurements during breaks were excluded from the analysis.
Nerve conduction studies
Pre-, mid-, and end-season NCS were carried out at the mink skinning facility. Post-season NCS took place at a hospital department of clinical neurophysiology. Only the dominant side was examined. On all four occasions, the same experienced technician performed NCS using Keypoint. NET system, version 2.11 (Alpine Biomedical, Skovlunde, Denmark). The temperature of the hands was kept at a minimum of 34º Celsius by the use of an electrical heater and heat pads. The NCS followed the standard of the department: median nerve motor studies by stimulation at the wrist and elbow and recording from m. abductor pollicis brevis; ulnar nerve motor studies by stimulation at the wrist and recording from m. abductor digiti minimi; sensory studies with antidromic technique by stimulation of the median and ulnar nerves at the wrist and recordings from digits 2 and 3 for the median nerve and from digit 5 for the ulnar nerve with an active ring electrode around the middle of the proximal phalanx referred to a ring electrode around the middle of the intermediate phalanx. Motor nerve conduction in the distal parts of the nerves was assessed by the distal motor latency (DML) and in the forearm of the median nerve by motor nerve conduction velocity (MNCV). Sensory nerve conduction was assessed by the conduction velocity calculated as the conduction distance divided by the sensory latency. Compared with the use of sensory latency, the use of sensory nerve conduction velocity (SNCV) has the advantage that anatomical landmarks can be used for electrode placement omitting the need of a standard distance between stimulation and recording electrodes (22). We used the age-specific reference values of the department to calculate z-scores for each participant, ie, deviations from the reference values expressed in standard deviations (SD) (23). A z-score was considered abnormal if larger than 1.96. The department’s electrodiagnostic criteria for CTS were that at least two of the three z-scores for median nerve DML, SNCV digit 2, and SNCV digit 3 were abnormal in the presence of normal ulnar nerve parameters. Cursors for latencies were set by the same investigator who was blinded to the order of the measurements.
Symptoms and disability
At pre-, end-, and post-season, the mink skinners completed a questionnaire which included a question on tingling sensations in the hand, the Katz hand diagram (24), the Levine questionnaire for the assessment of severity of symptoms and functional status in CTS (25), and the authorized Danish translation of the disabilities of the arm, shoulder and hand (DASH) questionnaire (26). We classified the Katz hand diagrams as “classic/probable”, “possible”, and “unlikely” CTS (24). The Levine questionnaire is side-specific and has two components: a symptom severity scale and a functional status scale with 11 and 8 items, respectively. Each item is scored from 1 (mildest) to 5 (most severe) and symptom and function scores are calculated as the mean for each scale (25). The DASH contains 30 items concerning the combined disability of both upper extremities and yields a score ranging from 0–100 with greater disability scoring higher (26).
Case definition
Our CTS case definition required that the department’s electrodiagnostic criteria were fulfilled (see above) and that clinical criteria were fulfilled in terms of a Katz hand diagram classified as “classic/probable” or “possible” (27).
Personal factors
For descriptive purposes, we collected questionnaire information on number of previous seasons with mink skinning, height, weight, smoking, and alcohol consumption. We calculated pack-years of smoking and transformed alcohol consumption to units per week, where one unit was defined as 12 grams of alcohol. Body mass index (BMI) was calculated as weight/height squared (kg/m2). We also had information on age.
Statistical analysis
To illustrate changes in NCS parameters over time, we plotted z-scores for each individual. We used paired t-test to evaluate intra-individual changes in NCS parameters and changes in Levine and DASH scores. There were no missing items in the Levine or DASH questionnaires. Two-sample t-test was used to evaluate differences between the mean numbers of minks skinned per hour in day and evening shifts across the whole skinning season. Data were analyzed using Stata 13 (StataCorp LP, College Station, TX, USA).
Results
Table 1 shows characteristics of the 11 male mink skinners who participated. One was left-handed, the others right-handed. One participant had previously had surgery for ulnar neuropathy at the elbow, none of the participants had ever had wrist surgery, and none of them had diabetes, rheumatoid arthritis, or thyroid disease. Outside the skinning season, all were students or had jobs that did not entail repetitive movements.
Table 1
Pre-season characteristics of the participating 11 male mink skinners. [SD=standard deviation; IQR=interquartile range]
Table 2 shows exposure characteristics of mink skinning based on goniometer measurements for six mink skinners during one day shift (mean recording duration 432 minutes) and surface EMG measurements for four (mean recording duration 427 minutes – for the remaining two, the measurements were lost because the electrodes loosened). Mink skinning was characterized by a median angle of wrist flexion/extension of 16º extension, a median velocity of wrist flexion/extension of 22 °/s, and force exertions of 11 % MVE.
Table 2
Wrist exposures during mink skinning. Simultaneous dominant-sided full-shift goniometer (N=6) and electromyography recordings (N=4) a. All participants were male. [SD=standard deviation; MVE=maximal voluntary electrical activity]
| Wrist exposures | Measures | Mean | SD |
|---|---|---|---|
| Postures | Angular distribution of flexion/extension (°) b | ||
| 10th percentile | -41 | 9 | |
| 50th percentile | -16 | 9 | |
| 90th percentile | 12 | 6 | |
| Non-neutral postures (% time) | 19.7 | 10.5 | |
| Movements | Median velocity of flexion/extension (°/s) | 22.4 | 3.4 |
| Repetitiveness (mean power frequency; Hz) | 0.43 | 0.04 | |
| No movement (% time <1 °/s) | 1.1 | 0.7 | |
| Exertion of force | Extensor activity (% MVE) | ||
| 10th percentile | 0.8 | 0.4 | |
| 50th percentile | 3.9 | 1.3 | |
| 90th percentile | 11.2 | 3.8 | |
| Extensor rest (% time <0.5 % MVE) | 6.9 | 3.4 |
During the measurements, the mean number of minks skinned per hour was 116. The mean for all the mink skinners across the whole season was 109 minks per hour with no significant difference between day- and evening-shifts (114 and 105 minks per hour, respectively; P=0.450). The skinning season lasted 22 calendar days and the 11 mink skinners worked a total of 220 days, ie, 20 days per participant on average. Four participants had a total of 4.5 sick days during the skinning season, none of which were related to upper-extremity symptoms.
Figure 1 shows the NCS values for each mink skinner from pre-season 10th November 2014 (N=11), through mid-season 24th November 2014 (N=10) and end-season 2nd December 2014 (N=11), to post-season 22nd December 2014 (N=2) and 14–15th January 2015 (N=7). All mink skinners had normal pre-season median nerve values. A total of nine skinners showed changes in the direction of median nerve conduction impairment during the skinning season and subsequent recovery. At end-season, five mink skinners had abnormally increased median nerve DML, and four and six had abnormally decreased SNCV from wrist to digit 2 and digit 3, respectively. Five fulfilled our electrodiagnostic criteria for CTS. One worker (depicted with gray lines and hollow squares in the online version of figure 1) had previously had a deep laceration of his 5th finger, resulting in ulnar nerve damage. There were no systematic changes of the ulnar NCS values.
Figure 1
Dominant-sided median and ulnar nerve distal motor latency (DML) and sensory nerve conduction velocity (SNCV) expressed in z-scores at pre- (Pre), mid- (Mid), end- (End), and post-season (Post). Each mark represents one measurement and each line one individual. Dashed horizontal lines show normal limits; at 1.96 in the DML panels and -1.96 in the SNCV panels. [APB=m. abductor pollicis brevis; ADM=m. abductor digiti minimi]
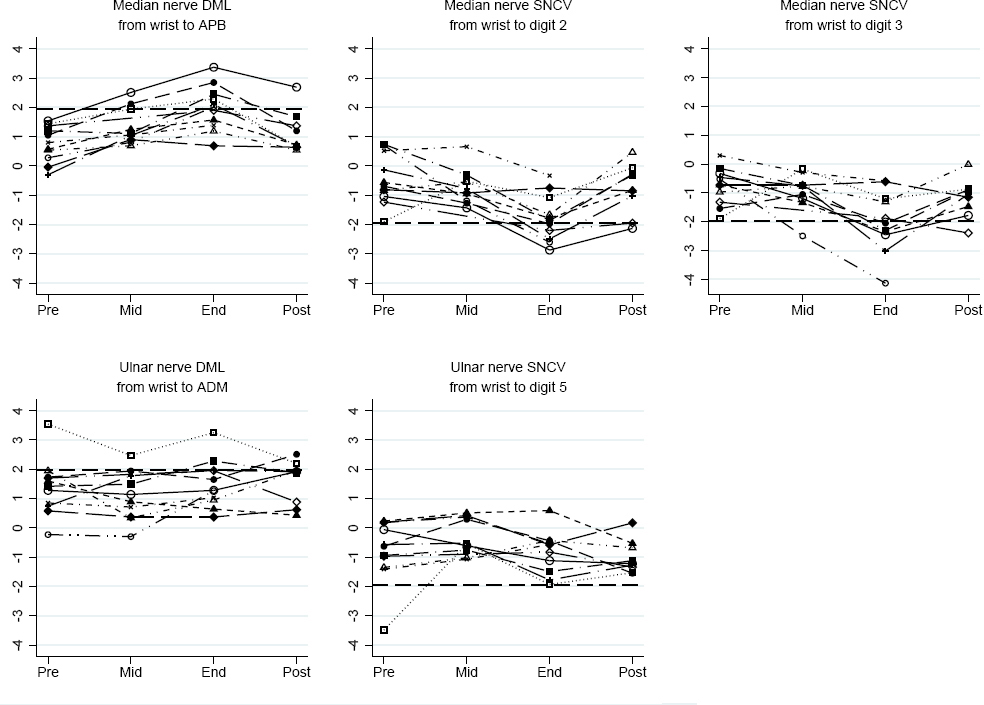
Table 3 shows mean NCS values at the four measurement occasions and mean paired differences from pre- to end-season and from end- to post-season. From pre- to end-season, there was an increase in median nerve DML and a decrease in median SNCV from digits 2 and 3; thus, sensory and motor changes occurred simultaneously. There were no changes in median MNCV from elbow to wrist. From end- to post-season, the changes reversed. There were no significant differences between pre- and post-season; results not shown. Between pre- and mid-season, the only significant change was an increase in median nerve DML (P=0.002). There were no significant changes for the ulnar nerve.
Table 3
Nerve conduction parameters at pre- (Pre), mid- (Mid), end- (End), and post-season (Post) and differences from Pre to End and End to Post. There are minor differences between “Difference End to Post” and the difference between “End” and “Post” due to two participants with missing post-season measurements. [ADM=m. abductor digiti minimi; APB=m. abductor pollicis brevis; 95% CI=95% confidence interval; DML=distal motor latency (ms); MNCV=motor nerve conduction velocity (m/s); SNCV=sensory nerve conduction velocity (m/s)]
At pre-season, 3 of the 11 mink skinners reported tingling sensations in their dominant hand. This number increased to 8 at end-season and returned to 2 out of 9 at post-season (2 of the mink skinners did not answer the post-season questionnaire). The corresponding numbers with hand diagrams that were classified as “classic/probable” or “possible” were 2, 8, and 2, and our case definition of CTS was fulfilled by 0, 4, and 0.
The Levine symptom score increased from 1.3 [95% confidence interval (95% CI) 1.0–1.7] at pre-season to 2.1 (95% CI 1.5–2.7) at end-season (P=0.022) and then returned to 1.3 (95% CI 0.9–1.7; P=0.012). All Levine function scores were around 1.1 with no significant changes (results not shown). The mean DASH score increased from 5.7 (95% CI -0.1–11.5) at pre-season to 10.4 (95% CI 4.3–16.6) at end-season (P=0.002) with a subsequent decrease to a post-season mean of 4.9 (95% CI -0.6–10.3, P=0.013).
Discussion
We found that 22 days of hand-intensive seasonal work led to impaired median nerve conduction in 9 of 11 mink skinners, 4 of whom fulfilled our case definition of CTS at end-season. The changes reverted to normal post-season. None of the mink skinners had abnormal pre-season NCS parameters, which indicates that median nerve conduction was not impaired by previous skinning seasons.
The study took advantage of a natural experiment provided by seasonal mink skinning, which allowed us to study nerve conduction and CTS symptoms in a targeted workforce with a highly standardized exposure pattern, a setup which has previously only been possible in experimental animal studies (13, 14). Even though the study population was small, the NCS changes were of a magnitude that enabled us to detect significantly decreased median nerve conduction and recovery. Since we used repeated NCS, the workers acted as their own controls, which minimized any confounding. A further strength was that the investigator who evaluated the NCS was blinded to the seasonal order of the examinations when he set the latency cursors.
We performed direct technical measurements of the exposures with equipment that has been used in several previous studies (17, 28–30). Regarding postures, work as a mink skinner was placed in the middle of the spectrum observed for repetitive industrial work with respect to wrist flexion/extension (28, 30), and the % time spent in non-neutral postures was lower than the value of 30% time reported for house painters in the only other study that has used this particular exposure measure (17). With respect to median velocity of wrist flexion/extension, values of 15–40°/s have been measured for occupational groups with repetitive industrial work, which places work as a mink skinner in the middle of the spectrum, comparable to fish processing and wood industry workers (laminate production and parquet slats sorting) and below meat cutters and poultry workers (28, 30). Work as a mink skinner showed a higher median velocity of wrist flexion/extension than work as a house painter (15.7°/s) (17) and office work (≤10°/s) (28). The 90th percentile for % MVE was 11.2 for the mink skinners, which was lower than values of 15–35% MVE which have been reported for other groups with industrial repetitive work (28, 30) and actually comparable to office work. We only achieved four EMG measurements and we cannot rule out that we underestimated this exposure. However, the mink skinners did not experience their work as force requiring and an investigator who observed the work process rated the intensity of exertion as a 2 (somewhat hard) using a scale ranging from 1 (light) to 5 (near maximal), corresponding to % MVE values of 10–29% (31).
The exposure measures in our study can be compared to exposure–response relationships based on studies that have used identical methods for exposure assessment (17, 28). Among house painters, who had median velocities of wrist flexion/extension within a limited range (14–17°/s), a Danish study of clinically diagnosed CTS suggested an adjusted incidence rate ratio for men of 1.15 per 1°/s (17). A meta-regression of data from eight previous Swedish studies of male workers with a wide range of median velocities of wrist flexion/extension (3–35°/s) showed an increase in the prevalence of clinically diagnosed CTS of 0.3% per 1°/s (28). According to our judgment, the mink skinners’ observed work pace would place them at a hand activity level of around 7 on the American Conference of Governmental Industrial Hygienists’ scale, which ranges from 0 (hand idle most of the time; no regular exertions) to 10 (rapid, steady motion/difficulty keeping up or continuous exertion) (32). An Italian study of CTS confirmed by NCS among manual and non-manual workers found a hazard ratio of 1.8 for hand activity levels between 5.1 and 8.5, when compared to levels between 1.0 and 3.0 (33), and a comparable North American study found a hazard ratio of 1.3–1.5 for hand activity levels >4 (34). Thus, exposure intensities comparable to those of the mink skinners have previously been related to an increased risk of CTS.
Even though the mink skinners’ wrist exposures were not exceptionally high as compared to other kinds of repetitive industrial work, nearly all of the participants exhibited decreases of nerve conduction across the carpal tunnel and a considerable fraction (36%) developed CTS. Previous cross-sectional studies that have used a case definition similar to ours have found a CTS prevalence of 6.3% among slaughterhouse workers (83% men) (35), around 8% among female supermarket cashiers (36), and 3% among construction workers (99% men) (37). The lower prevalence in those studies might be explained by a healthy worker survivor effect, modified work techniques developed over time, and biological adaptation to exposures. We do not know the course of impaired median nerve conduction in case of continued occupational mechanical exposures. The observed changes might resolve or increase to a more severe impairment with protracted recovery or even irreversibility; this remains to be studied.
Our findings agreed with the only other human study that we are aware of, which focused on the time course of median nerve impairment in relation to occupational activities (9). The proportion that displayed decreases of nerve conduction was not reported in that study, but animal studies have shown that the majority of the exposed individuals developed impaired median nerve conduction (13, 14). Our findings also showed that work-related impaired median nerve conduction may occur as rapidly in humans as animals. CTS in pregnancy is generally thought to be related to increased pressure in the carpal tunnel, and findings regarding CTS in pregnancy are in accordance with a short induction period and subsequent improvement (38–40). After surgery for CTS and even intra-operatively, rapid reversibility of NCS values has also been reported (41). Our study is unique in that it is the first to show that 22 days of repetitive industrial work can lead to impaired median nerve conduction and CTS with subsequent recovery. Quick reversibility suggests that other mechanisms than demyelination and axonal degeneration play a role in mild subacute CTS.
We have no reason to think that a replication of our study in other male or female populations with similar exposures would lead to substantially different results. For clinical practice, our findings implicate that patients who develop CTS in relation to a newly increase in occupational mechanical exposures can be informed that the condition is most likely reversible within weeks if the exposures are reduced. For national surveillance purposes, register information on CTS diagnosis and surgery may be used to identify job groups with high incidence rates, which suggest high exposure intensities. Within high-risk job groups, occupational health practitioners may use median NCS before and after workplace interventions to make sure that safe exposure intensity levels are reached.
In conclusion, this study took advantage of a natural experiment to evaluate median nerve function in relation to seasonal exposure to repetitive industrial work with moderate wrist postures and limited force exertion. The results showed that impaired median nerve conduction and CTS can develop in a considerable fraction of individuals during a few weeks of exposure and recover within weeks after exposure cessation.



