Evidence based on the scientific literature reviewed by several expert groups shows an association between moisture and mold-related exposure in buildings and the following respiratory outcomes: upper-respiratory tract symptoms, wheeze, cough, asthma symptoms, and the development of new asthma (1–3). At the time our study was being planned, the scientific evidence was limited for all of the aforementioned associations with moisture and mold exposure. The epidemiological evidence is still weak for new-onset asthma, but there are a few relevant studies (4). Finland experienced a sudden epidemic of mold-induced asthma in the beginning of the 1990s. According to the Finnish Register on Occupational Diseases, indoor air molds were the most frequently reported cause of occupational asthma (OA) in Finland in 2001–2002 (5). Also in other countries, moisture- and mold-induced disease has been recognized and is a matter of concern (1, 3, 6). In New York State occupational health clinics, poor indoor air quality or molds were suggested as causative agents in over one-fifth of the cases of new-onset OA (7).
Indoor air problems are frequent in subarctic countries where heavily insulated buildings are required, and people spend some 90% of their time indoors. The effects of any harmful emissions, such as chemicals and mold products, can be enhanced by poor ventilation. Moisture damages have been demonstrated to be exceptionally common in Finland (8). Indoor air-related symptoms tend to be long lasting (9–11). In a Swedish study on “sick building syndrome”, long-lasting building-related symptoms were found seven years after the diagnosis (12). According to our clinical experience, asthma related to damp and moldy workplaces is often persistent, and patients often experience substantially deteriorated health and well-being. In addition to asthma symptoms, patients have a variety of non-specific symptoms, including symptoms of the eyes, skin and upper airways, as well as headache, hoarseness, and fatigue (13).
The effect of asthma on quality of life (QOL) has raised scientific interest fairly recently. QOL is frequently impaired among patients with OA when compared with the QOL of those with asthma unrelated to work (14–16). The same has been demonstrated for patients with work-exacerbated asthma (WEA) (17, 18). Asthma has been shown to be associated with significant psychological morbidity, including depression and anxiety, which are also associated with poor asthma control and worse QOL (19, 20). In addition, the psychological impact on OA has been studied (15, 16, 21). A recent review (22) stated the need to assess psychological distress levels (anxiety and depression) and psychiatric disorders, particularly anxiety and somatoform disorders (eg, hypochondriasis), among patients with suspected or confirmed OA.
Very few studies have examined the QOL of patients with indoor air-related symptoms. An evaluation of respiratory morbidity in a water-damaged building in the northeastern United States found trends of increasing impairment in QOL with an increasing severity of respiratory symptoms (23).
We are not aware of any studies that have assessed long-term outcomes of work-related asthma in relation to damp and moldy environments. Our large material of diagnosed mold-induced cases of asthma offered a possibility to study QOL aspects in this group. Our study aimed to (i) evaluate the QOL of patients with OA induced by exposure to dampness and molds as compared with the QOL of patients with WEA and patients without asthma but symptoms related to the exposure; (ii) characterize factors influencing the QOL; and (iii) determine whether or not psychological factors, anxiety, depression, or somatoform disorders explain worse health outcomes in relation to building-related asthma.
Methods
Study participants
The design was a cross-sectional study of patients who had been examined at the Finnish Institute of Occupational Health (FIOH) in 1995–2004 on suspicion of an occupational respiratory disease. All of the examined 2200 patients had experienced work-related respiratory symptoms that manifested in damp and moldy indoor environments. At the time of the exposure, most of the patients had worked in office-like surroundings, schools, hospitals, or child daycare centers. The patients had been referred to FIOH from all over the country, mainly by occupational physicians or pulmonologists. FIOH is known in the Finnish healthcare system to be a center of expertise in the diagnostics and management of OA, occupational rhinitis, and other occupational respiratory disorders.
In the baseline examinations, 186 patients were diagnosed with OA. The diagnoses were based on workplace exposure to indoor air molds, asthma that started during the exposure, the work-relatedness of the asthma symptoms (appearing or worsening at work and improving away from work), and at least one positive work-related, pulmonary function test result. As a consequence of the compensation policy of that time, the latter was most often a positive specific inhalation challenge response to a commercial fungal extract (5, 13). Occasionally, work-related changes in serial peak flow measurements sufficed for the diagnosis of OA. If skin prick tests or specific immunoglobulin (Ig) E measurements showed sensitization to molds, the result was used to confirm the diagnosis. The absence of conflicting findings was a prerequisite for OA.
Altogether 726 patients were classified as having WEA; their asthma symptoms were exacerbated by workplace exposures. The clinical tests fulfilled the criteria of asthma but were not eligible for the diagnosis of OA. The pulmonary function evidence of asthma was (i) significant improvement (≥15% and ≥200 ml) in the forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) in response to short-acting bronchodilating medication in a bronchodilator test or (ii) a repeated daily variation of ≥20% or improvement of ≥15% (and ≥60 l/min) in response to short-acting bronchodilating medication in a 2-week diurnal measurement of peak expiratory flow (PEF).
For the rest of the patients (N=1288), there was no objective clinical evidence of asthma in examinations performed at FIOH or earlier examinations. They either had asthma-like symptoms (cough, dyspnea, or wheeze) without asthma or work-related upper-airway and mucosal symptoms (like nasal congestion, rhinorrhea, or hoarseness). All of the patients had accompanying symptoms (like eye irritation, fatigue, headache, fever or chills, or dermal symptoms).
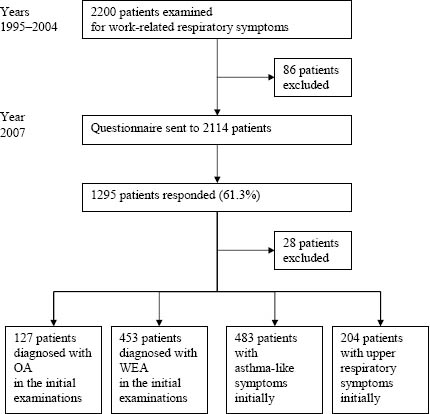
In the beginning of 2007 (3–12 years after the examinations), the patients were followed up by a questionnaire. On referral to FIOH, building dampness was suspected to have caused the symptoms of all patients. FIOH investigations found that a specific agent at work had induced the symptoms of 66 patients (eg, methacrylates in dental work or flours in kitchen work). We excluded these 66 patients with a definite other-than-building-related cause for their symptoms. In addition, 20 patients died during the follow-up. Thus the questionnaire was sent to 2114 persons, and 1295 participated (61.3%). All participants signed written informed consent. From the present analyses, we also excluded the 28 respondents who were diagnosed with alveolitis in the baseline examinations.
Thus the study population consisted of 1267 respondents. In the baseline examinations, 127 of the respondents had been diagnosed with OA, and 453 patients with WEA; 483 respondents suffered from asthma-like symptoms but did not have clinical asthma and 204 had only mucosal or upper respiratory symptoms (figure 1).
Follow-up questionnaire
In addition to demographic and other background data, the questionnaire included sections on current employment status, QOL, anxiety and depression, somatization, hypochondria, asthma medication, and current symptoms.
For health-related QOL, we used the well-validated Short Form Health Survey 12-item questionnaire (SF-12) (24) that measures physical and mental dimensions of health. It allows for the calculation of the following two summary scores: (i) a physical component score (PCS) comprised of questions on physical functioning, role limitation due to physical health problems, bodily pain, and general health; and (ii) a mental component score (MCS), including questions on vitality, social functioning, role limitation due to emotional health problems, and mental health. We also asked the respondents to evaluate their QOL on a visual analogue scale (VAS) of 0–100, which is a single-item instrument with good validity in measuring QOL (25). It is simple to perform and relies on the patient’s ability to form an overall judgment of QOL.
For psychological status, the widely used 14-item Hospital Anxiety and Depression Scale (HADS) was employed (26). HADS is a brief screening instrument. It was selected because it avoids any reference to physical symptoms. Seven questions relate to anxiety and seven to depression, with total scores for both subscales in the range of 0–21. A value of ≤7 is interpreted as nonclinical, 8–10 indicates possible clinical relevance, and values of ≥11 show important relevance.
Tendencies towards somatization were measured with a 12-item somatization subscale (SCL SOM) from the Hopkins Symptom Checklist (SCL-90) (27). SCL SOM is widely used and screens for multiple physical symptoms, focusing on cardiovascular, gastrointestinal, and other systems with autonomic mediation. The Finnish version of the SCL SOM has been validated (28). To measure the cognitive or emotional dimension of somatization, hypochondriasis, we used the 7-item version of the Whiteley Index (Whiteley-7) (29). It reflects the patient’s health anxiety, beliefs, and fears of illness and the attribution of physical sensations to physical illness. On both scales, the responses on symptoms or beliefs were rated on a 5-point Likert scale (ranging from “not at all” to “very much”).
The respondents were asked to mark a list for the presence or absence of their current chronic diseases or physician-diagnosed injuries. The conditions included accidental injury, musculoskeletal disease, cardiovascular disease, mental disorder, endocrine or metabolic disease, neurological or sensory disease, malignancy, and other disease (30). Respiratory diseases were excluded. Co-morbidity was classified as 0, 1 or ≥2 chronic conditions.
We enquired about the current need for asthma medication to estimate asthma severity. The respondents were asked to name the pharmaceutical products they regularly use to treat asthma. The need for asthma medication was grouped as (i) no need for regular asthma medication, (ii) inhaled steroid alone, (iii) inhaled steroid combined with a long-acting beta-agonist or leukotriene antagonist, and (iv) a combination of all of the three medicine groups. We also enquired about the number of oral steroid bursts needed to control asthma during the past year with the following possible responses: (i) no need during the past year, (ii) once a year, (iii) twice a year, or (iv) more often.
The prevalence of current respiratory symptoms was estimated by rating each symptom (cough, dyspnea, and wheeze) on a scale of severity ranging from 1 (not at all) to 5 (very much). Ratings of 1–3 were considered nonsignificant (score of 0), and only ratings of 4–5 (much or very much) were taken into consideration (score of 1).
Statistical analysis
Our data included both categorical and continuous variables. We applied the chi-square test when examining the differences in categorical variables among the patient groups. The differences between the means of the continuous variables were compared using the one-way analysis of variance (ANOVA). After these preliminary studies, we began to build models using the PCS, the MCS, and the VAS as outcome variables. All of our outcome variables were handled as continuous, and linear regression models were applied. As some explanatory variables were categorical and some continuous, the analysis of covariance (ANCOVA) was applied. Our model building strategy was to add independent variables step by step into the model. Only the results of the last models with estimates, standard deviations (SD), and P-values are presented.
We used the classified number of oral steroid bursts as an indicator of asthma severity and built a model by adding the indicator into the models. Only two asthma groups with 580 patients were included in these models.
A P-value of <0.05 was considered to indicate statistical significance. All of the analyses were performed using the SAS 9.1 (SAS Institute Inc, Cary, NC, USA). The Ethics Committee of the Hospital District of Helsinki and Uusimaa approved the study.
Results
The study population is described in table 1. Compared with the non-respondents, the participants were older [mean age 54.3 (SD 9.2) years versus 52.0 (SD 9.5) years], more often female (87% versus 84%), and more often diagnosed with OA (10% versus 6%). The frequency of WEA at baseline was similar for the participants and the non-respondents. For the study population, the mean time from the initial examinations to the point of follow-up with the questionnaire was 8.0 (SD 2.8, range 3.0–12.0) years. At the time of the follow-up, there were no significant differences in age or smoking habits in the comparison between the groups of OA, WEA, asthma-like symptoms, and upper-respiratory symptoms (table 1). A total of 60% of the patients had never smoked. The patients with WEA had an atopic history more often than those with OA or no asthma. Both asthma groups reported coincident chronic diseases or injuries more often than the non-asthmatics (table 1). Altogether, 88% of the respondents were white-collar employees (eg, teachers, nurses, office workers, and administrators) and 12% were blue-collar employees (eg, cleaners, kitchen helpers, and doormen).
Table 1
Patient characteristics at the time of the follow-up questionnaire. [OA=occupational asthma; WEA=work-exacerbated asthma; SD=standard deviation; NS=not significant]
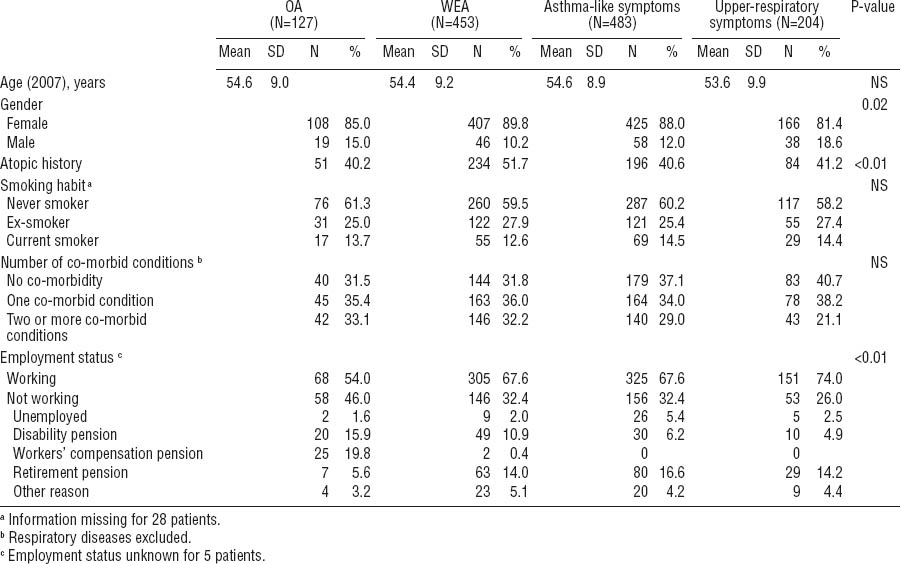
Of the OA group, a statistically significant proportion of the respondents were not working at the time of the follow-up (table 1). Altogether 41% of the patients with OA received a pension, either due to disability (workers’ compensation pension or disability pension) or old-age retirement (63–68 years). In the other groups, pension was not as common (26% of the WEA patients, 23% of the patients with asthma-like symptoms, and 19% of those with upper respiratory symptoms).
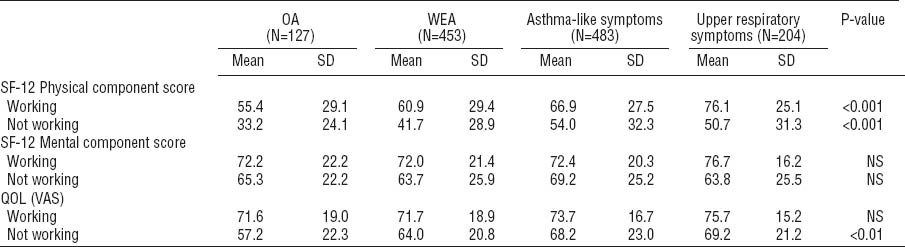
Table 2 shows the scores of the generic QOL, depression, anxiety, somatization, and hypochondria indicators. Statistically significant differences were observed between the four groups according to the asthma and symptom status at baseline. The OA patients had the lowest scores, indicating worse QOL, in all of the QOL domains. For the MCS component of the SF-12, the differences between the groups were smaller and not significant. When the asthma groups were combined, 33% of all asthmatics had an important or possible clinical relevance for an anxiety disorder versus 37% of the respondents in the non-asthma groups. Correspondingly for depression, 13% of the asthmatics and 11% of the non-asthmatics had an important or possible relevance for an anxiety disorder. Surprisingly, patients with OA seemed to display less anxiety than the other groups (table 2). However, the result was not statistically significant. The scores of the somatization subscale (SCL-12) did not differ significantly between the groups, whereas the Whiteley-7 index for hypochondria showed statistically significant differences.
Table 2
Mean scores for the quality of life (QOL) domains and psychological scales, according to asthma or symptom status at baseline. In all of the QOL domains, a higher value indicates a better QOL. [HADS=Hospital Anxiety and Depression Scale; OA=occupational asthma; NS=not significant; SD=standard deviation; SF-12=Short Form Health Survey 12-item Questionnaire; VAS=visual analogue scale; WEA=work-exacerbated asthma.]
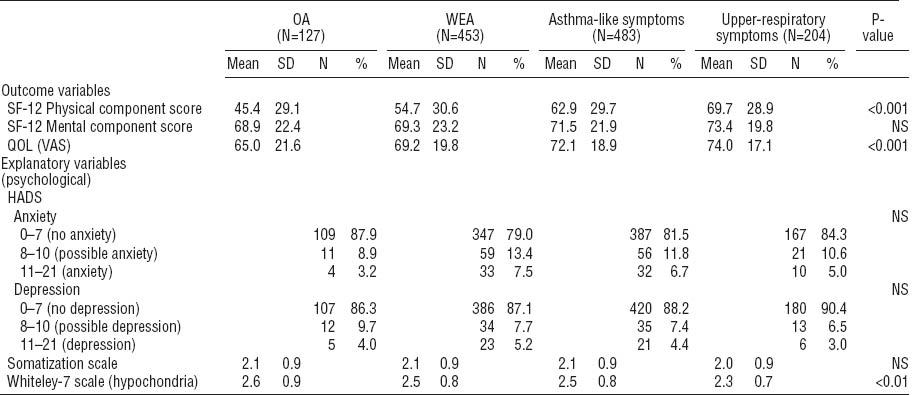
Table 3 presents the scores of the QOL domains for those who were working at the time of the follow-up and those who were not. Among those who were not working, the persons with OA had the lowest scores for QOL on the PCS component of the SF-12 and the VAS scale.
Table 3
Mean scores for the quality of life (QOL) domains for those currently working or not working, according to the asthma or symptom status at baseline. In all QOL domains, a higher value indicates a better QOL. [OA=occupational asthma; SD=standard deviation; SF-12=Short Form Health Survey 12-item questionnaire; VAS=visual analogue scale; WEA=work-exacerbated asthma.]
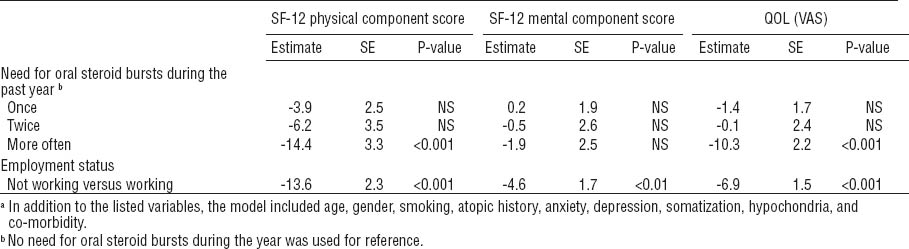
In a multivariate linear regression analysis, the group status was associated with QOL with respect to the PCS but not the MCS component of the SF-12 (table 4). An association could also be shown for the overall QOL measured by the VAS scale. The differences in the QOL scores were compared between each group. For the PCS component of the SF-12, the difference between the asthma-like and upper respiratory symptoms groups was not statistically significant (P=0.4). Between all the other groups, the differences were statistically significant (P<0.001). For the MCS component of the SF-12, there were no statistically significant differences between the groups, whereas the differences were statistically significant for the VAS scale when the OA group was compared with each of the other groups (P<0.05). Not working was a determinant for worse QOL in regard to all three QOL dimensions. Adding anxiety, depression, somatization, and hypochondria into the model did not remove the differences between the groups.
Table 4
Multiple linear regression analysis for the quality of life (QOL) domains [Short Form Health Survey 12-item Questionnaire (SF-12) and visual analogue scale (VAS)]. All of the variables were included in the model.a [OA=occupational asthma; SE=standard error; NS=not significant; WEA= work-exacerbated asthma.]
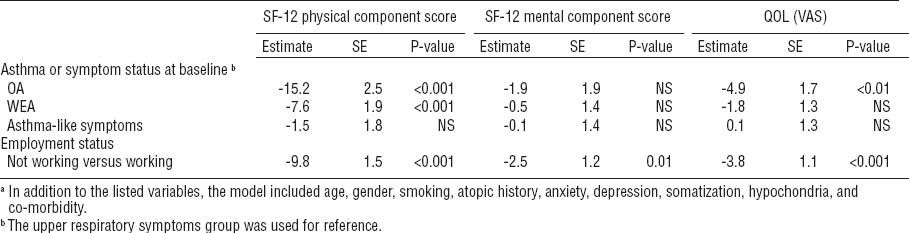
The need for regular asthma medication (P<0.001) and the need for oral steroid bursts to control asthma (P<0.001) was higher among patients with OA than those with WEA (table 5). A worse QOL (measured by the PCS component of the SF-12 or VAS scale) was associated with a higher need for asthma medication (P<0.001 for both scales) and a more frequent need for oral steroid bursts (P<0.001 for both scales). Significant cough and dyspnea were more prevalent among the OA patients than WEA patients: 27% versus 17% (P=0.01) and 28% versus 17% (P<0.01), respectively. For wheezing, the difference was not statistically significant (P=0.6).
Table 5
Current need for asthma medication and the need for oral steroid bursts during the past year in the occupational asthma (OA) and work-exacerbated asthma (WEA) groups.
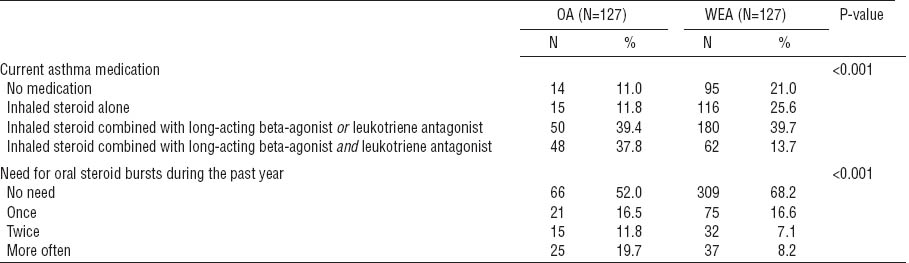
In a multivariate model including the OA and WEA groups, frequent oral steroid use was a strong determinant for a poorer PCS component of the SF-12 and overall QOL on the VAS (table 6). When regular asthma medication was included in the model as an explanatory variable, it was found to explain worse QOL with respect to the PCS (P-value <0.01), but not regarding the other QOL dimensions. Oral steroid use and regular asthma medication could not be added to the same model because of their strong mutual correlation.
Table 6
Multiple linear regression analysis for the quality of life (QOL) domains [Short Form Health Survey-12 Questionnaire (SF-12) and visual analogue scale (VAS) in the occupational asthma (OA) and work-exacerbated asthma (WEA) groups only. All of the variables were included in the model.a [NS=not significant; SE=standard error.]
Discussion
Our study showed that a high proportion of patients with OA caused by indoor air dampness and molds had impaired QOL 3–12 years after their initial diagnosis when compared with patients with WEA and patients with no asthma but symptoms related to the exposure. In a multivariate model adjusted for age, gender, smoking, atopic history, co-morbidity, and psychological factors, the differences between the groups remained for the physical but not the mental component of QOL. Not working was a strong determinant of deteriorated QOL in all of the dimensions. Anxiety, depression, or somatoform disorders did not remove the impact of OA and employment on QOL. Among the asthma groups, the persistence and severity of the disease were strong determinants for a worse QOL physical component.
In general, the results corroborate our clinical impression that a large proportion of patients with OA in relation to exposure to moisture and mold-damaged workplaces have long-standing limitations in everyday life and remain symptomatic and unable to work. Our study has wider relevance as a considerable number of asthma cases are attributable to building dampness and mold (31), even though they are not generally recognized as OA in many countries. The study indicates that QOL is deteriorated at least in a subgroup of patients with asthma related to workplace dampness. Malo et al’s study (14) was one of the first articles on QOL and OA. They investigated persons with OA two or more years after the exposure had ended. In comparison to persons with non-OA, the OA group showed greater impairment of QOL (14); this finding corresponds to our current results.
The follow-up was based on self-reported data, which is one of the limitations of the study. Because of the large size of the study population, a clinical re-examination of the patients was unfeasible. The same was true for a thorough investigation of home and workplace conditions at the time of the follow-up. On the other hand, the large number of subjects included is a strength of the study. FIOH acts as a reference institute in the diagnostics of occupational diseases in Finland, and therefore a large number of patient cases accumulated nationwide. Thus the study subjects were derived from the same source population, though some selection bias cannot be ruled out. The non-respondent analysis showed that the OA patients were more likely to participate in the study. There is, however, no reason to believe that OA patients with a poor QOL would have been more prone to respond than those with a good QOL. Even if there would have been a difference in the response rates in relation to QOL, it would apply to the participants in each group, and therefore we believe this potential for bias is limited.
Our finding that those who were employed had the best QOL scores has earlier been shown for workers diagnosed with OA induced by Western red cedar (32). In our previous study conducted in the same source population, six months after the diagnosis of OA, 58% had returned to work (13). Now, at the follow-up 3–12 years later, the employment status was nearly unchanged, with 54% of the respondents being currently employed. The proportion of employed persons appears surprisingly low, as the majority of our patients with OA were teachers, nurses, office workers, and the like, who should not become disabled from work when adequately medicated and removed from exposure to moist and moldy environments. Retirement as the reason for unemployment was conspicuously more frequent among the OA patients. By comparison, those who had asthma-like symptoms without asthma were more often without a job than the OA and WEA patients.
We had expected that the OA patients would be better off than the other groups due to a liberal compensation system for occupational diseases. In Finland, OA is compensated through the statutory accident compensation system, whereas WEA is not. Compensation covers, for example, medical expenses, income replacement, and vocational rehabilitation. Benefits for occupational diseases are usually better than those of non-occupational diseases, which are covered by other forms of social insurance. However, the OA patients clearly had a lower QOL than all other groups of patients. They did not seem to have profited from the legal benefits associated with an ascertained OA diagnosis.
As estimated by the use of asthma medication, the asthma symptoms of the OA patients were both more persistent and severe than those of the WEA patients. A large proportion of the OA patients were using heavy medication still 3–12 years after the diagnosis. Only 11% of the OA patients did not use inhaled steroids, and the need for oral steroid bursts was clearly greater (table 5). Both parameters correlated well with QOL – the more the medication, the worse the QOL. This finding tallies well with the results of a study by Miedinger et al (16), in which objectively measured disease activity seemed to be the principal determinant of QOL among 73 patients with OA 2 years after their diagnosis.
There may be various individual and environmental reasons for the persistence and severity of the disease. To avoid exposure, the options are building remediation or change to an alternative work environment. Moisture and mold damage in buildings has been excessively common in Finland (8), and often other deficiencies worsening the quality of indoor air, such as poor ventilation, occur in connection with moisture problems. Successful repairs are not always easy to achieve, and therefore symptoms can persist among sensitive persons. In some buildings, the inhabitants may exhibit persisting symptoms despite various environmental improvements (33). A 3-year follow-up of a 97-person cohort in a water-damaged building found that substantial remediation did not result in an improvement in respiratory health, as reflected in symptom scores, overall medication use, spirometry abnormalities, or sick leave (34). The results of a Canadian study indicated that, after mold exposure, at least a subgroup of mold-exposed persons had long-term respiratory symptoms that could not be explained by asthma (6). Their initial symptoms could be attributed to allergic symptoms, but additional symptoms similar to findings in connection with the “sick building syndrome” persisted despite removal from or remediation of mold exposure (6).
Mental factors should not be ruled out as a reason for persistent symptoms and low QOL. When we adjusted for depression and anxiety, the differences in QOL between the groups remained. For our patients, the rates of anxiety and depression were lower than those of Björnsson et al (35) who reported positive associations between building-related symptoms and anxiety and depression, as measured by the HADS. In addition, compared with the results of previous studies on asthmatics, psychological distress was lower than expected among our patients (15, 20, 21, 35). Yacoub et al (15) reported significant rates of depression and anxiety, close to 50%, 2 years after the cessation of exposure among persons with sensitizer-induced OA. In a study by Malo et al (21), the rates were up to 40% 3–22 years after the diagnosis of acute irritant-induced asthma. The rates seem to be higher for OA than for non-OA, as Lavoie et al (20) observed rates of 23% for anxiety disorders and 20% for depressive disorders in a study on tertiary care patients with asthma.
Neither somatization nor hypochondria explained the differences in QOL between the groups. Earlier, a tendency towards somatization was shown to be associated with symptoms related to the “sick building syndrome” (37), which is the reason we included somatization in our study. The mean scores of the SCM SOM scale for a community sample of the Finnish population in a study by Holi et al (28) were clearly lower than those of our patients, and in an outpatient sample of psychiatric Finnish patients the scores were higher. Higher SCL SOM scores (38), as well as higher hypochondria scores (39), have been shown to be associated with female gender, and females were overrepresented in our material. Similarly, there is an association with somatic diagnoses (38), as all of the symptoms on the SCL SOM scale may be reflections of a physical illness. Our results did not point to any greater degree of somatization among our OA patients compared to the other groups. The hypochondriac features measured by a self-rating scale – the Whiteley-7 index – did not seem to be high in any group; yet a somewhat lower value was scored by the upper-respiratory symptoms group.
In our study, VAS as an overall measure of QOL proved to correspond with the physical dimension of the SF-12. Many studies of QOL in the assessment of asthma have used disease-specific questionnaires, but generic measures, like the Short Form 36 (SF-36) or the brief version SF-12, are the most commonly used health-related QOL instruments. We used a generic measure in our study because we wanted to have non-asthma groups as a reference. A disease-specific and a generic QOL instrument showed fairly good internal consistency in assessing asthma patients in a general population (40). A single-item VAS assessing QOL was found to be a valid measure when compared with multi-item questionnaires, and it showed moderate-to-high correlations with indicators of physical, psychological, and social aspects of QOL (25).
A differentiation between OA and WEA caused by exposure to building dampness and molds is difficult to achieve, and there are several reasons for possible misclassification (13). Nevertheless, we found an impaired QOL among the patients diagnosed with OA caused by workplace dampness and molds when they were compared with patients with WEA or symptoms only in corresponding environments. Various and probably many unknown factors may contribute to an impaired QOL, involving factors outside the workplace. The stressful nature of an OA diagnosis may play a role (21), although psychological distress did not explain the impact of OA on QOL in our study. OA seemed to be more severe than WEA. Clinical follow-up studies are needed to investigate the severity of asthma in OA induced by dampness and molds and its impact on QOL objectively. The findings indicate that comprehensive measures for rehabilitation and effective asthma management, including exposure control, are needed for promoting good QOL among these patients.