Since an increasing number of patients are likely to return to work after diagnosis and treatment completion, there is an increasing recognition of the short and long-term impact of cancer and both its physical and psychosocial consequences on employment during the last years (1–13). Unfavorable cancer and treatment consequences include a variety of physical and functional disabilities, and psychological distress that may adversely affect a patient’s work ability, work satisfaction, as well as employment status (14–17). Since work has the potential to help patients regain a sense of meaning, normalcy and being valued, returning to work may comprise a range of positive consequences for the recovery and the psychological well-being (18, 19).
Previous research suggested that cancer increases the risk of unemployment among survivors compared to healthy controls (20–22). On average, 63.5% of cancer survivors (range 24–94%) return to work (17). Overall, studies indicate a steady increase of return to work (RTW) from on average 40% at six months post diagnosis to 62% at 12 months, 73% at 18 months, and 89% at 24 months. Factors significantly associated with a greater likelihood of being employed or RTW include perceived employer accommodation, flexible working arrangements, counseling, training and rehabilitation services, younger age, higher education, male gender, a lower physical symptom burden, cancer remission, shorter length of sick leave, and continuity of care (7, 14, 17, 23–28).
However, although important findings have emerged in previous studies on work and employment among cancer survivors (7, 29, 30), only limited knowledge exists about RTW after rehabilitation taking into account the impact of demographic characteristics, cancer and treatment-related physical factors, psychosocial as well as of work-related aspects of employment and RTW. The Word Health Organization (WHO) (31) has defined rehabilitation as “the use of all means aimed at reducing the impact of disabling and handicapping conditions and at enabling people with disabilities to achieve optimal social integration”. Medical rehabilitation programs in Germany are provided to cancer patients according to the overall aim defined by the WHO and the International Classification of Functioning, Disability and Health (ICF) (31). Thus, the (re-)integration of individuals with disabilities, chronic health conditions, diseases, and handicaps into society and working life is one important aspect of rehabilitation by eliminating or reducing the impact of chronic illness and disability. The aim is to maintain a patient’s optimal physical, sensory, psychological, and social functional levels. Rehabilitation also serves to prevent an impending disability or the aggravation of existing physical damages.
Based on social laws in Germany, cancer patients have a legal right to participate in at least one rehabilitation program after the completion of primary cancer treatments (32). Traditionally, cancer rehabilitation programs are mainly carried out in in-patient settings in specialized rehabilitation clinics. Access to cancer rehabilitation programs is usually facilitated by hospital doctors and social workers immediately after completion of the primary treatment (“follow-up rehabilitation”). However, cancer rehabilitation at a later stage during the course of cancer treatment is also provided. Rehabilitation costs are covered mainly by the pension and health insurances. A cancer rehabilitation program lasts three weeks following a multidimensional therapeutic approach that includes patient education, exercises, and physical therapy to regain physical fitness and vitality, relaxation training, psychosocial counseling, and psychosocial support groups to enhance coping skills as well as individual psychotherapy.
This prospective longitudinal study aimed to (i) identify the employment rate 12-months after cancer rehabilitation, (ii) explore the work situation and experienced work changes, and (iii) identify demographic, medical, functional, psychosocial, and work-related predictors of the likelihood and time period of RTW.
Methods
The study received research ethics committee approval. All patients provided written informed consent prior to participation. Consecutive patients were recruited from four cancer rehabilitation facilities and assessed at the beginning (t0) and end of rehabilitation (t1), and 12 months after rehabilitation (t2). Inclusion criteria comprised (i) age 18–60 years, (ii) the capability to complete study measures, and (iii) the absence of permanent invalidity and early retirement. Since the old age pension in Germany generally becomes effective at 65 years, the age limit of 60 years was chosen to enable patients to have a sufficient time period left to return to work.
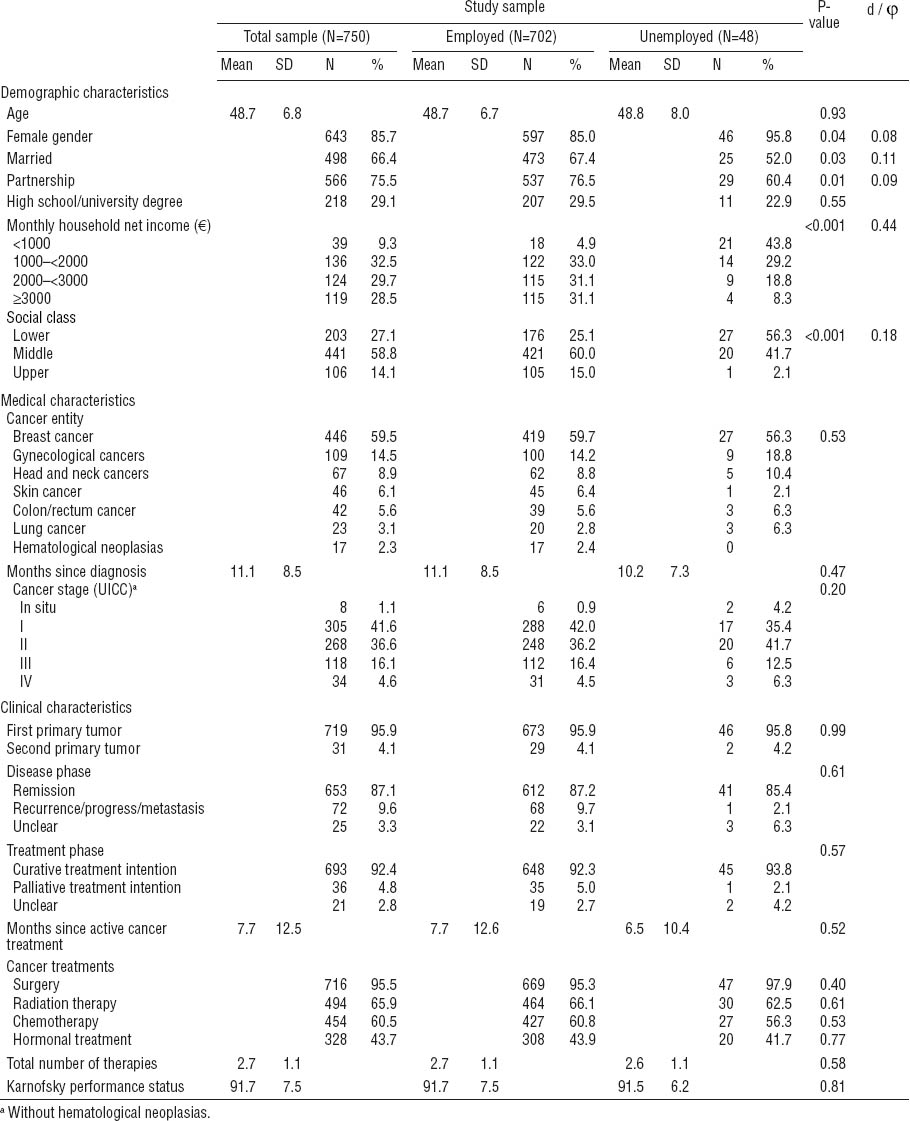
A total of 1148 patients were enrolled at t0 (75.5% participation rate), whereas 372 patients declined to participate. Among those who participated at t0, 1060 completed the questionnaires at t1. At t2, questionnaires were mailed to an eligible 994 patients (36 patients had moved to an unknown address and 30 had died), 750 (75%, 65% of the total sample) of whom returned questionnaires that could be evaluated (figure 1). Table 1 presents baseline sample characteristics.
Figure 1
Enrollment of cancer survivors (t0=beginning of cancer rehabilitation, t1=end of a 3–4 week cancer rehabilitation, t2 =12 months after cancer rehabilitation)
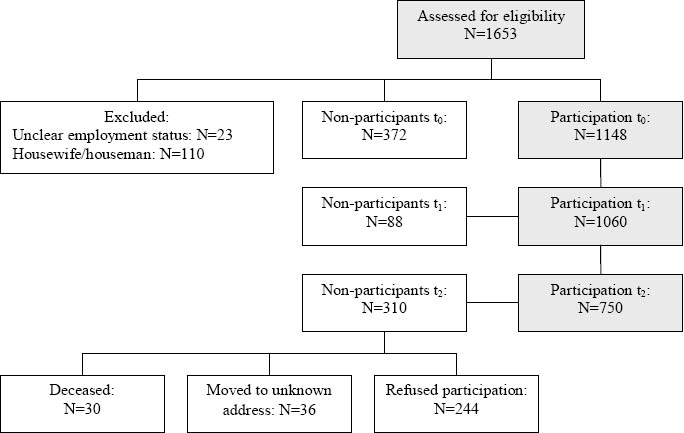
Table 1
Baseline demographic and medical sample characteristics. [SD=standard deviation; UICC=International Union against Cancer]
Non-responder analyses
At t0, participants and non-participants differed in terms of age [mean 48.5, standard deviation (SD) 7.2, years versus mean 50.4, SD 6.1, years] (P<0.001) (d=0.3) and male gender (16% versus 22%) (P=0.007) (φ=0.07). No group differences in cancer entities were observed. At t2, non-participating patients were more likely to be male (P=0.008) (φ=0.09), widowed (P=0.03) (φ=0.10), and have head and neck or lung cancer (P=0.005) (φ=0.14). Participants at t2 were found to be significantly less depressed (P=0.01) (η2=0.007) and had a lower level of fear of cancer recurrence (P=0.009) (η2=0.007).
Study variables and measures
The main outcomes for this study were employment at 12 months after rehabilitation (M=23.1 months after cancer diagnosis) and time until RTW. Employment was defined according to a patient’s positive response to the question “Are you currently working?” Time until RTW was measured in weeks after rehabilitation.
Demographic information was obtained at baseline consisting of standardized questions concerning (age, gender, marital status, and employment history). Education, monthly household net income, and occupational position were used to calculate a 3-factor social status index (33). Medical information was collected at baseline [cancer entity, months since diagnosis, cancer stage as defined by the International Union against Cancer (UICC), clinical characteristics and disease phase]. In addition to the baseline Karnofsky performance status (34), physicians estimated the degree of functional impairment using cancer-entity-specific physical functioning scales. The Karnofsky status is a performance measure for rating the ability of a somatically ill person to perform usual activities. A person is evaluated on a score of 0–100, where 0=dead and 100=normal, no complaints, no signs of disease. Using cancer-entity-specific physical functioning scales, the physician measures the limitations specific for each tumor entity such as shoulder mobility or lymphedema in breast cancer, incontinence in genital or colon cancers, swallowing problems in head and neck cancers or dyspnea in lung cancer. Pain intensity during the last week was evaluated using the Brief Pain Inventory (BPI) (35).
The psychosocial and work-related variables were further assessed at baseline. Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS) (36). Fear of cancer recurrence was measured using the 12-item short version of the Fear of Recurrence Questionnaire (FoP-Q-SF) scored on a 5-point Likert scale ranging from 1=never to 5=very often (37). The Short-Form Health Survey assesses dimensions of quality of life (QoL): Here, the two summary scores for physical (PCS) and mental health (MCS) were calculated. Higher scores indicate better QoL (38). The Illness-Specific Social Support Scale (ISSS) measures the degree to which partners/friends provide positive support (eg, “listened to you”) or act in a non-supportive way (detrimental interactions) (eg, “tried to change the way you’re coping with your illness in a way you didn’t like”). Items are scored on a 5-point Likert scale ranging from 0=ever to 4=always (39).
Occupational and work-related characteristics were assessed using questions and brief questionnaires developed and psychometrically evaluated by Bürger et al (40). Occupational information included (i) professional status, (ii) work ability and periods of sick leave absence, (iii) 10 items about job requirements (eg, many work responsibilities, high pressure of competition, tight schedules) answered on a 4-point Likert scale ranging from 1=almost never to 4=quite often (Cronbach’s a=0.87), and (iv) 12 items about work satisfaction answered on a 7-point Likert scale ranging from 1=not satisfied at all to 7=totally satisfied (Cronbach’s a=0.92). Self-perceived work ability was evaluated on a 5-point Likert item ranging from 1=totally limited to 5=not limited at all; self-perceived employer accommodation was evaluated on a 5-point Likert item ranging from 1=not at all to 5=extremely. Perceived threat of job loss was measured using single-item questions (No/Yes).
Statistical analysis
In order to identify significant predictors of RTW, demographic, medical, functional, psychosocial, and work-related factors were entered separately (block-wise) into a multivariate hierarchical logistic regression analysis against the outcome variable “RTW”. Step-wise backwards elimination was used, testing each candidate variable for removal using Wald statistic. Before testing the regression model, we performed correlations (Pearson and Spearmans correlation coefficients) for all predictor candidates between variables in one block and between candidate variables and the outcome criteria. In order to identify significant predictors of the time period of RTW, candidate predictor variables were entered into a Cox’s proportional hazards model to calculate hazard ratios. To provide an estimate of the magnitude of the group differences, Cohen’s standardized effect size (φ, d, η2) was calculated. Two-tailed significance tests were conducted using a significance level of P<0.05.
Results
Baseline occupational characteristics
At the beginning of the rehabilitation program, 702 patients (93.6%) were employed and 48 (6.4%) were unemployed. Among the employed, 54.9% were on physician-classified sick leave (usually the primary care physician). The majority of the working participants worked as employees (75.1%), 18.4% were workers; 5.6% were self-employed, and 1.0% worked as civil servants. The mean duration of sick leave within the 12-months period prior to the rehabilitation program was 150.6 (SD 107.4, range 1–365) days. The majority of patients (84.5%) were motivated either to return to work or be re-employed after rehabilitation.
RTW and re-employment 12 months after rehabilitation
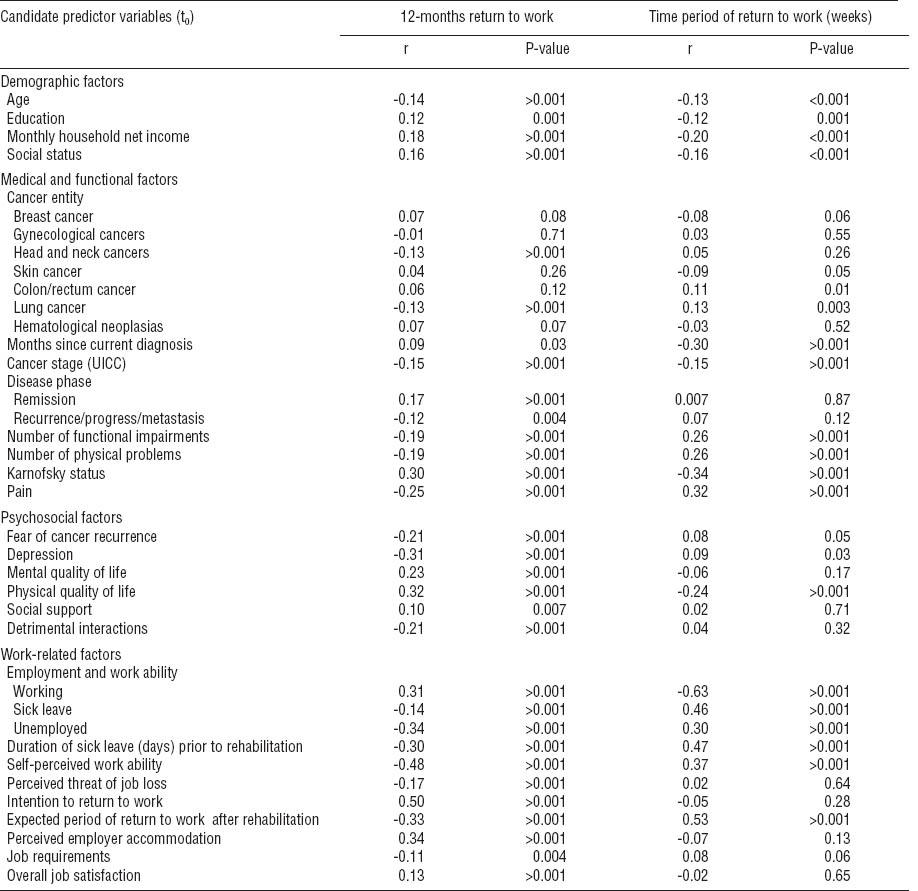
At t2, 568 patients (75.7%) had returned to work or were re-employed. The percentage of patients who returned to work was highest among participants not on sick leave at baseline (92.4%). Twenty-one percent of the patients who were unemployed at baseline managed to get employed at a follow-up time point (table 4). Patients who returned to work or were re-employed were slightly younger [mean 48.2, (SD 7.0) years versus mean 50.5 (SD 6.1) years] (P<0.001) (η2=0.021). No gender differences were observed. The highest percentage of patients who returned to work was observed among employees (97.2%), and the lowest percentage among workers (66.4%) (P=0.02) (φ=0.12). No group differences were found in clinical characteristics. However, among patients with a higher UICC cancer stage (P<0.001) (φ=0.18) and palliative treatment, a lower percentage (38.9%) returned to work or were re-employed compared to patients with curative treatment (78.1%) (P<0.001) (φ=0.20). Also, the highest percentage of patients who did not return to work was observed among patients with lung cancer (43%) and head and neck cancers (58%). Among patients with cancer progress or metastatic cancer, a significantly lower percentage (38.9%) returned to work or got re-employed compared to patients in remission (78.6%) (P<0.001) (φ=0.17).
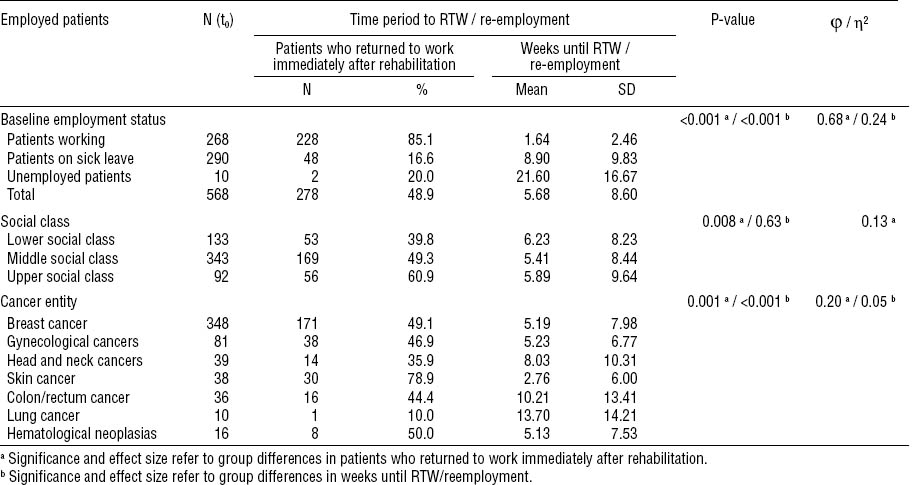
RTW time period and re-employment after rehabilitation
Forty-nine percent of patients returned to work immediately after rehabilitation. The mean time until RTW or re-employment was 5.7 (SD 8.6, range 1–45) weeks. The highest percentage of patients who returned to work immediately were among those who were not on sick leave at baseline, belonged to the upper social class, and had skin cancer (table 2).
Work situation at follow-up
Among the 568 patients who returned to work, the majority (81.2%) returned to their former position and workplace; 46% worked full-time. More women (77.8%) worked part-time compared to 22.2% of men (P<0.001) (φ=0.25). Fifty-two percent of the patients were on sick leave absence at least once after rehabilitation for an average duration of 62 days.
Predictors of RTW – logistic regression model
Before testing the logistic regression model, we performed correlations between variables in one block for all predictor candidates. In candidate predictor variables with an intercorrelation of r≥0.60, one variable was removed from the regression analysis in order to avoid multicollinearity. The following variables were removed: marital status, treatment phase (curative versus palliative treatment intention), months since active cancer treatment, total numbers of therapy, anxiety, distress, and the expected period of RTW. Table 3 presents the correlations between the final candidate predictor variables and the outcome variables.
Table 3
Correlations between candidate predictor variables (t0) and outcome variables. [UICC=International Union against Cancer.]
The first block entered into the multivariate hierarchical logistic regression model consisted of demographic factors (table 4). The variables education and social status were excluded from the regression model, whereas age and monthly household net income remained in the model (Nagelkerke’s R²=0.07) (P<0.001).
Table 4
Multivariate logistic regression model for the identification of significant predictors of 12-months (re-) employment after rehabilitation. [OR=odds ratio; SD=standard deviation; SE=standard error; 95% CI=95% confidence interval]
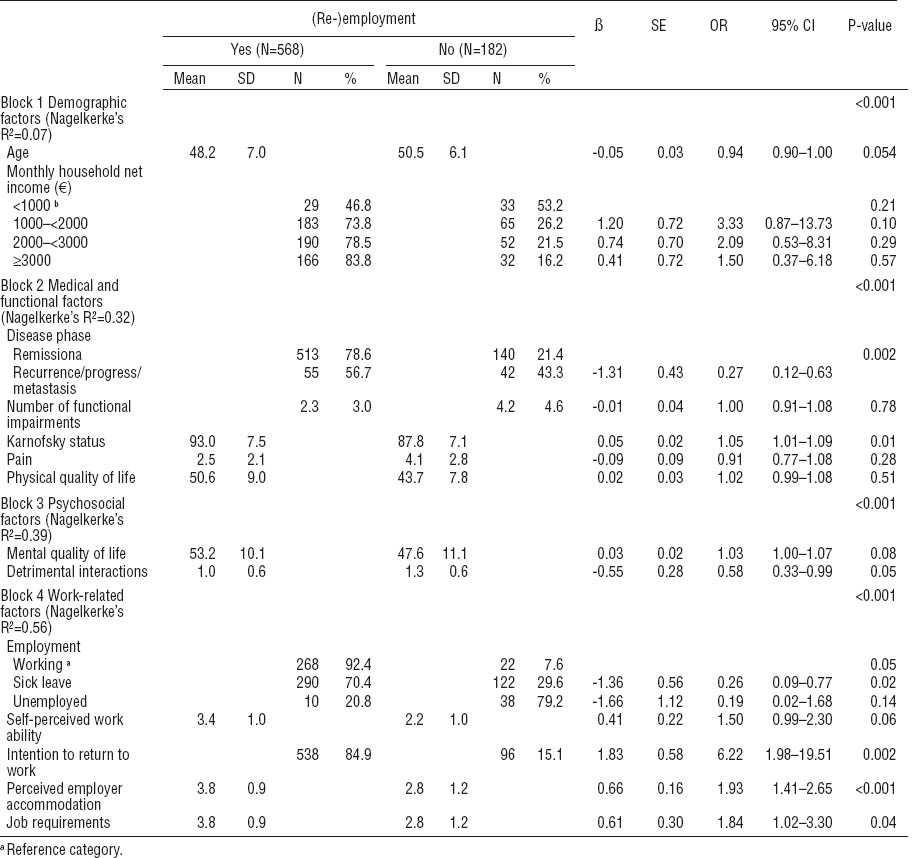
The second block entered consisted of medical and functional factors. The variables cancer entity, months since current diagnosis, UICC cancer stage, and number of physical problems were excluded from the regression model (Nagelkerke’s R²=0.32) (P<0.001). Patients with cancer recurrence or cancer progress had a significant lower chance to return to work compared to patients in cancer remission.
The third block entered consisted of psychosocial factors. The variables fear of recurrence, depression, and social support were excluded from the regression model (Nagelkerke’s R²=0.39) (P<0.001). Individuals with a higher amount of problematic social interactions were less likely to return to work.
The forth block entered consisted of work-related factors. The following variables were excluded from the regression model: duration of sick leave (days) prior to rehabilitation, perceived threat of job loss, expected period of RTW after rehabilitation, and overall job satisfaction. Patients who intended to return to work at baseline were more than six times as likely to do so (P<0.001). Also, patients who perceived their employer as being cooperative and accommodating of their cancer were more likely to return to work. Although the variable job requirements showed a negative correlation with RTW, it also emerged as a positive predictor for RTW. This is likely an effect of suppression since high job requirements are positively associated with RTW among individuals in the higher social class but negatively associated with RTW among patients in lower social class. When the variable is entered as a single factor into a regression analysis, it remains as a negative predictor of RTW [ß=-0.41, OR=0.67, 95% confidence interval (95% CI) 0.48–0.92, P=0.01]. The explained variance of the total model (Nagelkerke’s R²) was 0.59 (P<0.001).
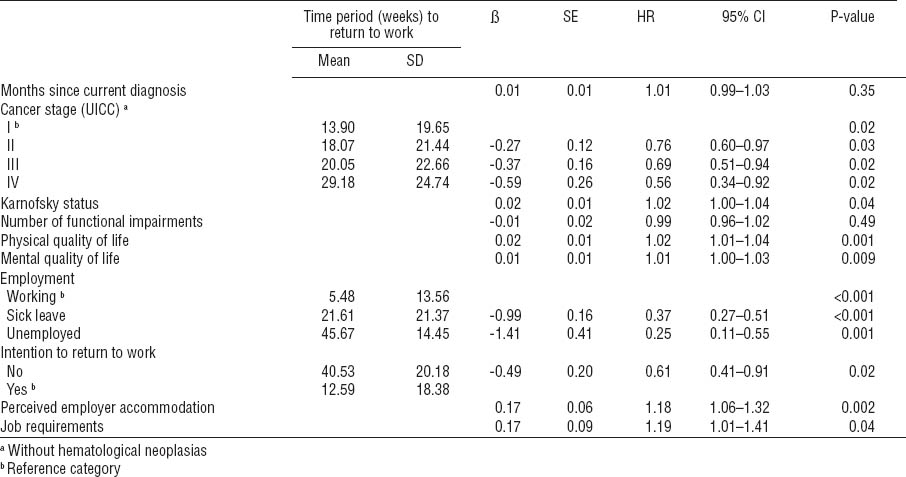
Predictors of time period to RTW
The following variables remained in the model as significant predictors: UICC cancer stage, Karnofsky index, physical and mental quality of life, employment characteristics, intention to return to work, perceived employer accommodation, and job requirements (overall model fit - 2 log likelihood = 3858.2; χ2[total model] = 175.6, df = 13, P<0.001) (table 5).
Discussion
The majority of patients (85%) were motivated either to return to work or be re-employed after rehabilitation. These findings are consistent with results indicating the significance of work and the strong motivation to continue work during treatment or return to work after treatment completion among survivors (41). The exploration of work characteristics revealed that slightly more than half of participants (55%) were on sick leave at baseline indicating a high physical and/or psychological symptom burden at the beginning of the rehabilitation program. In our study, the employment rate was 76% on average 23 months after diagnosis and considerably higher compared to findings showing that overall, about 63.5% of cancer patients (range 24–94%) manage to return to work (17). However, comparable results (73%, range 64–82%) have been found at 18 months after the cancer diagnosis among breast cancer patients (14) and among samples with mixed tumor entities (1, 8, 25). Concordant with previous studies showing work changes in 8–17% of cancer survivors, we found similar results (25, 30).
Both the absence of sick leave and belonging to a higher social class at baseline were associated with a significant higher rate of employment at follow-up. Belonging to a higher social class is related to work environments that may provide more favorable working conditions in terms of flexibility, a lower degree of manual and physically exhausting work, better earnings, and better living conditions. Accordingly, it has been stated that employees with cancer or other persistent health problems generally need some flexibility at various aspects and times at work (42). Atkinson et al (43) and Gudbergson et al (44) pointed out that living conditions include the social indicators that stimulate social inclusion and reduce social exclusion. These indicators are economy, education, employment, health, housing, and social participation.
The highest percentage of patients who did not return to work was observed among patients with lung cancer (43%) and head and neck cancers (58%), among patients with advanced cancer stage, progress or metastatic cancer, and palliative treatment intention. The adverse effect of cancer progress and poor physical functioning on RTW has been shown in several previous studies (7, 23, 27). Corresponding to previous research, we found re-employment significantly associated with younger age and a professional status as an employee (24, 27, 45).
Our regression analyses findings emphasize the importance of volitional factors for RTW. Patients who expressed their intention to return to work at the beginning of the cancer rehabilitation program were six times more likely to do so compared to patients who did not intend to return to work. Our findings show that the patient can best predict RTW at an early stage of the rehabilitation process. However, so far only limited knowledge exists about motivational and volitional factors and its association with demographic, family and work-related aspects (46, 47). Occupational motivation or skepticism towards RTW should be carefully assessed at the beginning of rehabilitation programs and during the joint establishment of rehabilitation aims between the patients and the professional team (48). Furthermore, factors influencing occupational motivation among cancer survivors need to be understood in more detail.
The strong influence of work-related aspects next to medical factors such as disease phase confirm the current state of the literature with regard to factors positively and adversely influencing RTW among cancer survivors (7, 14, 23, 25, 26, 45). Consistent with the literature, the perceived employer accommodation indicated a fairly positive attitude towards employment, work-related support, and necessary job changes (14, 27, 49) and emerged as a significant predictor for RTW.
In our study, analyses also demonstrated that the patients who were working at baseline were more likely to return to work after cancer rehabilitation and were more likely to do so at an earlier stage than patients on sick leave or – unsurprisingly – unemployed patients. Self-perceived work ability, in contrast, did not emerge as a significant predictor for RTW. The relevance of perceived work ability has been emphasized in a number of studies (3, 15, 44, 50–53). However, only limited knowledge exists about the association between perceived work ability and sick leave absence.
Furthermore, we found that detrimental social interactions were inversely associated with RTW. Patients with problematic social interactions in their personal environment might also lack social skills at the workplace or might have fewer personal resources to effectively adapt to the challenges of being a cancer survivor in the work environment.
The variable “high job requirements” showed a negative correlation with RTW. However, it also emerged as a positive predictor for RTW likely due to suppression effects. Our findings emphasize that more research is needed particularly to investigate the kind of job requirements (such as having plenty of work or high time pressure) that might lead to early retirement, unemployment, or a higher probability to RTW. Our results point toward the fact that high job requirements are positively associated with RTW among individuals in the higher social class but are negatively associated with RTW among patients in the lower social class. Individuals belonging to a higher social class are more likely to have better working conditions such as flexible work, responsibility, and a considerable amount of decision-making freedom that might compensate the high workload.
Although this prospective study includes a large sample size compared to previous research, this study has several methodological limitations. Both the initial and follow-up non-response lead to a bias in several sociodemographic and psychosocial outcome variables of interest in this research. With regard to the generality and interpretation of the findings, a sample bias must be considered toward: (i) female gender, (ii) younger age, (iii) being in a cancer rehabilitation program, (iv) cancer entities associated with a better physical health status and prognosis, and (v) better psychological well-being. Given this bias, our findings with regard to employment might overestimate the degree to which cancer patients return to work or stay employed. Nevertheless, systematic differences between participants and non-participants were small in view of effect sizes and at least partially a consequence of the relatively large sample size. Although 86% of the patients were women, the overall large sample size including 107 men justifies gender analyses; however, gender did not correlate with employment status and therefore was excluded from further regression analyses.
Another limitation is that due to the allocation process mainly regulated by the German pension insurance, it was not feasible to randomize the study sample. Despite the fact that cancer rehabilitation programs are provided to every cancer patient in Germany, our sample consists only of patients who use the services provided. This might lead to a bias towards a sample with high physical and psychosocial impairments. Furthermore, the inclusion of a matched control group could not be realized since most patients with rehabilitation needs are referred to a rehabilitation program. Thus, this study could not determine possible effects of the rehabilitation program on RTW.
The (re-)integration of cancer survivors into working life is one important aspect of participation according to the ICF (31). Rehabilitation programs are important not only for the physical and psychosocial recovery, but for the labor market reintegration of patients. Profound understanding of cancer and treatment-induced impairments and their impact on daily activities and work is an essential basis for the development of better educational, rehabilitative, and occupational interventions in cancer care (54). A better understanding of cancer and treatment-induced physical, cognitive and psychosocial treatment consequences related to work-related problems will help to develop interventions and educational programs for patients, healthcare professionals, and employers to better address the professional needs of individuals with cancer.