Acute myeloid leukemia (AML) is a cancer of blood and bone marrow. It accounts for approximately 25% of all leukemias among adults (1).
While the etiology of AML is poorly understood, it has been linked to genetic disorders, physical and chemical exposures, radiation exposure, chemotherapy, and viruses (2, 3).
There is a general consensus that benzene can cause AML in humans (4–8). Some previous studies suggested an association between exposure to solvents and AML (9–11).
The aim of this study was to assess the relation between occupational solvent exposure and AML.
Methods
The current study employed a case–control design nested in the Nordic Occupational Cancer Study (NOCCA) cohort. The NOCCA cohort consists of 14.9 million individuals from Finland, Iceland, Norway, Denmark, and Sweden who participated in population censuses in 1960, 1970, 1980/1981, and/or 1990. As we had no access to the individual records from Denmark, their data were not included.
Occupational information was obtained from computerized census records from 1960 and later censuses in Sweden and Norway and from 1970 and later censuses in Finland. In Iceland, the only computerized census record was available for 1981 census. Personal identity codes were used to link census records with the data from cancer registries and national population registries for information on cancer, death, and emigration. Each person was followed-up until the date of emigration, death or 31 December of the following years: 2005 in Finland, 2004 in Iceland, 2003 in Norway, 2003 in Denmark, and 2005 in Sweden. A detailed account of the NOCCA cohort was given in Pukkala et al (12).
All incident AML cases diagnosed from 1961–2005 were extracted from the NOCCA cohort. Five controls per case were randomly selected among persons who were alive and free from AML on the date of diagnosis of the case (hereafter the “index date” of the case–control set). Cases and controls could have a history of any cancer other than AML and were matched for the year of birth, sex, and country. Persons with minimum age of 20 years at index date, and having occupational information from at least one census record, were included in the present study.
For each case and control, the exposure to solvents and other occupational factors was estimated based on conversions of occupational codes to quantitative amounts of exposures with the NOCCA job exposure matrix (JEM). National experts from the Nordic countries developed the NOCCA JEM on the basis of the Finnish JEM (13). It covers more than 300 specific occupations, 29 exposure agents and 4 periods: 1945–59, 1960–74, 1975–84, and 1985–94. Exposure agents are characterized by the proportion of exposed (P) and the mean level of exposure among the exposed persons (L) in a specific occupation and time period. The NOCCA JEM was described in detail in Kauppinen et al (14).
We quantified exposure to benzene, toluene, perchloroethylene, methylene chloride, trichloroethylene, and 1,1,1-trichloroethane as individual solvents; and aliphatic and alicyclic hydrocarbon solvents (ALHC), aromatic hydrocarbon solvents (ARHC), chlorinated hydrocarbon solvents (CHC), and other organic solvents (OSOL) as grouped solvents. In addition to this, we quantified exposure to 16 other agents which were considered as potential co-factors in the analysis (table 1).
Table 1
Individual/grouped solvents and co-factors with measurement units. [PPM= parts per million of agent; mg/m3=milligram of agent in cubic of workroom air; f/cm3=fibers of asbestos in cubic centimeter of workroom air; µg/m3=microgram of agent in cubic meter of workroom air; µmol/l=micromoles of lead in liter of blood; mSv=annual equivalent radiation dose in millisieverts.]
The procedure for quantifying cumulative exposure was as follows. For each occupational code, a corresponding value of the product of proportion and level of exposure (P×L) from NOCCA JEM file was assigned. This value was then multiplied by employment period (T) in years during which the subject was in that occupation. The procedure was repeated for all exposure factors.
Employment period was assumed to start at the age of 20 and end at 65 years. If there were different occupational codes in census records for a given person, the individual was assumed to have changed occupation in the middle of the known census years. In such a case, the exposure history of the persons consisted of more than one P×L×T value. Cumulative exposure for these individuals was estimated by summing up all of their P×L×T values over the entire working career. Assuming that AML develops over a number of years, and recent exposures are less relevant than those which took place in the past, we conducted main analyses in which all exposures that occurred during the last ten years before the index date were not counted.
We estimated hazard ratios (HR) and 95% confidence intervals (95% CI) for each solvent by conditional logistic regression. We selected values corresponding to the 50th and 90th percentiles of cumulative exposure distribution among all exposed case/control subjects as cut-off points for categorization. We defined exposure values of 0–50th percentile inclusive as “low”, 50–90th percentile inclusive as “moderate”, and >90th percentile of exposure distribution as “high”. Individuals with 0 exposure were used as the reference group. Test for trend was performed for a dose–response relationship between exposure factors and AML.
Variable selection for the final main-effect models was based-on the “purposeful covariate selection” procedure (15). Benzene and toluene were highly correlated with ARHC. Therefore, we selected two alternative main-effect models. In Model 1, we included benzene and toluene but not ARHC; and in Model 2, we included ARCH but neither benzene nor toluene. All other solvents were included in both models, and they were also adjusted for ionizing radiation and formaldehyde as co-factors. The results from both models were similar. Therefore, we present only the results of Model 1, except for the ARHC results, which can only come from Model 2.
Analyses stratified by age and sex was conducted to explore potential age- and sex-specific interactions with exposure. Age at index was categorized into two broad levels: <50 and ≥50 years, because only a small proportion of subjects (11–12%) were <50 years (table 2).
Finally, all analyses were done with different lag-time assumptions (0, 3, 5, 7, 10, and 20 years).
Results
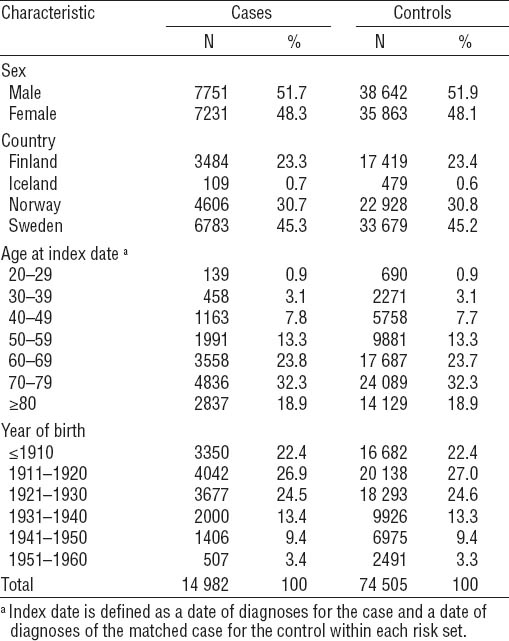
Altogether, 15 332 cases and 76 660 controls were identified during the study period (table 2). Of these, 350 cases (2.3%) and 2155 controls (2.8%) were excluded because they were either <20 years or had no occupational record. The proportion of male subjects was 52%, and 45% of the cases and controls were from Sweden. About 90% of case/controls were born before 1940, and the mean age at AML diagnosis was about 67 years (table 2).
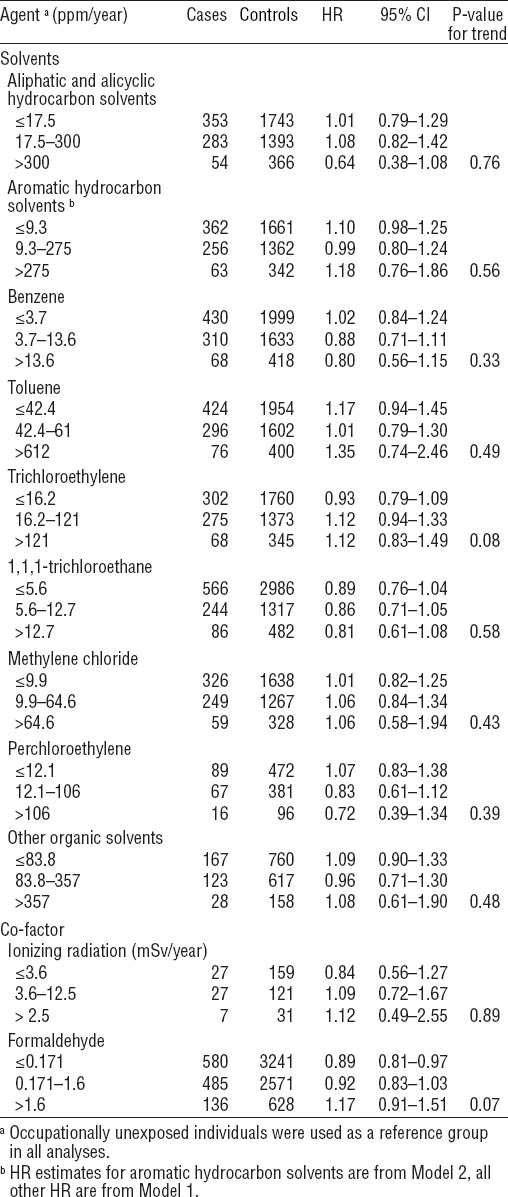
We did not observe statistically significantly increased risk or dose–response relationship for any solvent exposure and AML (table 3). The highest risk estimate was observed for toluene levels >90th percentile of exposure distribution (HR 1.35, 95% CI 0.74–2.46). Risk estimates associated with exposure to high levels of ARHC and high and moderate levels of trichloroethylene were slightly elevated. Similarly, a small increase in risk estimates for exposure to high levels of ionizing radiation and formaldehyde were also observed.
Table 3
Hazard ratios (HR) and 95% confidence intervals (95% CI) of acute myeloid leukemia associated with exposure to solvents and other co-factors. [Ppm=parts per million; mSv=millisieverts].
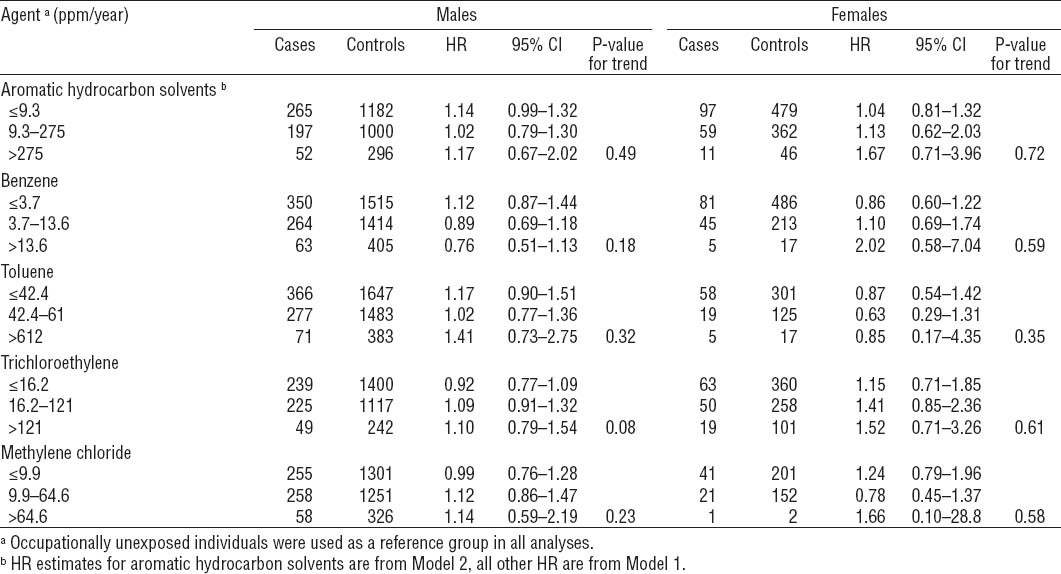
Sex-specific risk estimates associated with exposure to high levels of toluene were higher among men than women (table 4). In contrast, HR estimates for ARHC, benzene, methylene chloride, and trichloroethylene were more pronounced among women than men.
Table 4
Sex-specific hazard ratios (HR) and 95% confidence intervals (95 % CI) of acute myeloid leukemia associated with exposure to solvents with sex-specific variation of HR estimates. [ppm=parts per million].
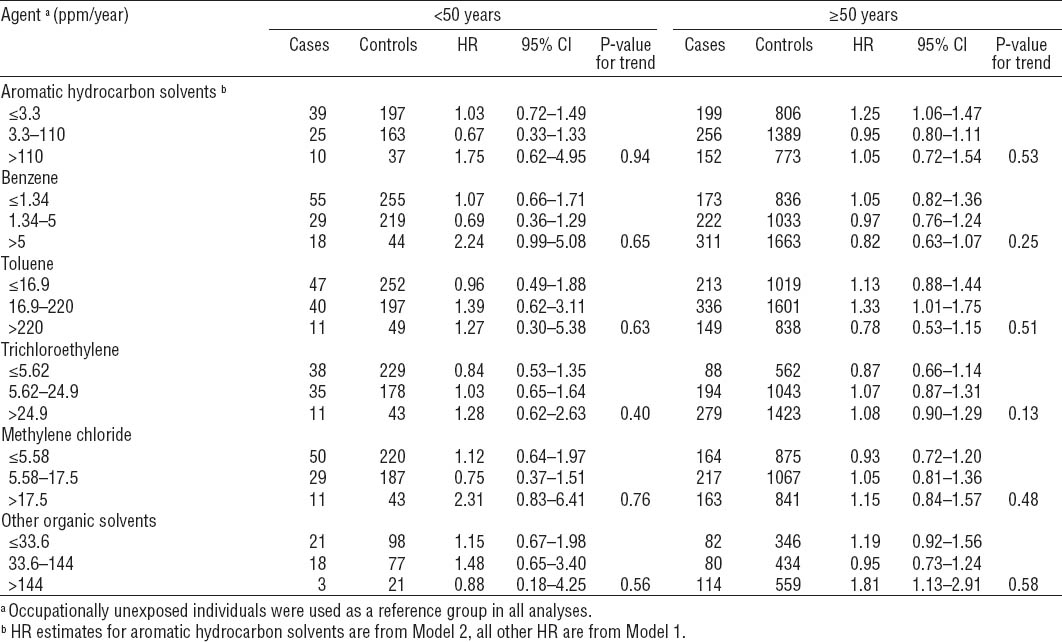
Analysis stratified on age showed that HR estimates of AML associated with exposure to some solvents might be higher in younger ages (table 5). For example, the risk of AML was more than two-fold among benzene- and methylene-chloride-exposed individuals <50 years, whereas it was close to one among those ≥50 years.
Table 5
Age-specific hazard ratios (HR) and 95% confidence intervals (95% CI) of acute myeloid leukemia associated with exposure to solvents with age-specific variation of HR estimates. [ppm=parts per million].
Analysis with different lag-time assumptions showed that HR estimates for toluene were 1.86 (95% CI 1.06–3.28) with 0 lag-time and 1.62 (95% CI 0.89–2.96) with 20 year lag-time. HR for ARHC and trichloroethylene remained elevated from 0–10-year-lag-time and decreased towards null with 20-year-lag-time (data not shown).
Discussion
The results from this study showed some suggestive evidence for an association between AML and solvent exposure but not consistently in both genders. There was non-significant excess risk for toluene, ARHC, and trichloroethylene. We did not observe an association between exposure to benzene and AML unlike many earlier studies (4–8).
The evidence for an increased risk of AML from exposure to organic solvents other than benzene is inconsistent. The recent studies by Kaufman et al (10) and Strom et al (11) suggested an increased risk of AML following solvent exposure. However, both studies had limitations with exposure assessment. In the former study, the authors combined benzene, gasoline, and other solvents into a single group due to a small number of exposed subjects. In the latter, exposure information was collected via personal interviews and was, therefore, subject to a recall bias. In an Italian multicenter case–control study no association between exposure to solvents and AML was found (16). Excess risk of AML from benzene exposure could be identifiable already at 10 ppm/year exposure levels according to Vlaanderen et al (8). The estimated cumulative benzene exposure in our study exceeded 13.6 ppm/year for 68 cases and 418 controls (table 3). These numbers are so high that our study is unlikely to lack power and miss an effect should one exist in our data.
Observed age and sex-specific variation in risk estimates could result from effect of physiological differences by age and sex on pharmacokinetics (absorption, distribution, metabolism, elimination) of solvents (17–21). However, we cannot exclude the possibility of chance findings.
When interpreting our results, the following limitations need to be considered. Because this study was based on general populations of the Nordic countries, only a small percentage of study population had considerable exposure to solvents. This constrained our choice of cumulative exposure categorization. Another potential limitation of the present study is exposure misclassification, which may arise from two sources. First, the generic JEM has a poor sensitivity and a failure to account for heterogeneity in exposure levels within jobs (22, 23). Work history data is a second factor likely to contribute to exposure misclassification. Individual work histories were based on census records that are a snapshot of a job held by individual at the time of the census. The data did not provide information on the changes of the job or tasks during the entire working career of an individual. In this study, we assumed that an individual held his/her occupation until the mid-year between two censuses. Finally, we could not control for smoking and genetic factors that have previously been linked to AML (24–27). However, lung cancer risk was not elevated in the majority of occupational groups with solvent exposure (12), suggesting that the smoking rate among solvent-exposed individuals was unlikely to be higher than the general population. In addition, genetic factors are very rare and unlikely to be related to solvent exposure. Therefore, smoking and genetic factors could have only a little, if any, confounding effect on our estimates.
To our knowledge this was the largest study to assess the relationship between occupational exposure to solvents and AML, which covered the general populations of four Nordic countries. By linking occupational records to the NOCCA JEM, we were able to generate quantitative exposure estimates for 26 work-related agents. It enabled us to explore exposure–response relations beyond the analysis of crude categorization (“exposed” versus “non-exposed”) or analysis by occupational groups. In addition, we were able to control for exposure to multiple agents and variation of exposure levels over time.
In conclusion, this study did not provide a clear evidence for an association between occupational solvent exposure and AML. There was some indication for an excess risk among those exposed to toluene, trichloroethylene, ARHC.