The publication of the results of the US National Lung Screening Trial (NLST) (1) has led to great interest in the possibility that screening for lung cancer should be performed among individuals assessed to be at high risk for lung cancer. The criteria for high risk in the NLST trial were based entirely on age and smoking history. Since then there have been several attempts to refine the criteria based on data collected either from the Prostate, Lung, Colon and Ovary Trial (2) that evaluated chest x-ray screening or the NLST itself (3). However, these attempts did not include occupational exposure to asbestos as a risk factor for lung cancer. In this paper, we report our approach to this, based upon the lung module of the Canadian Risk Management Lung Cancer (CRMM-LC) model (4, 5), supported by the Canadian Partnership against Cancer. Although mesothelioma is induced by asbestos exposure, there is no evidence that screening reduces mortality from the disease. Therefore, mesothelioma will not be considered as an endpoint in this paper or in the risk calculations that follow.
The context of our calculations is the expectation that policy decisions will be taken in Canada to introduce provincially-based organized screening programs for lung cancer based upon the best available evidence. Experience has already been gained in Canada in introducing LDCT screening in many centers, with process measures that measure up well to those exemplified by the NLST (6).
Determining the eligibility of LDCT screening among asbestos-exposed individuals is complex because there is no full agreement on the risk from asbestos exposure on its own, or with tobacco. As a result, it is difficult to define “at risk” individuals. This uncertainty over the level of increase in risk of lung cancer caused by asbestos exposure may derive from different contexts of study. Thus the asbestos, asbestosis, and cancer: consensus report of the 1997 Helsinki Criteria for diagnosis and attribution indicated that relative risk (RR) is roughly doubled for cohorts exposed to asbestos fibers at a cumulative exposure of 25 fiber-years or with an equivalent occupational history, at which level asbestosis may or may not be present or detectable (7). Fiber-years is defined as exposure level (fibers/cm3) × exposure period (years): <10 fiber-years (low exposure); 10–24 fiber-years (moderate exposure), and ≥25 fiber-years (high exposure) (8). In contrast, Gustavsson et al’s case–control study in Sweden (9) found more than a doubling of risk of lung cancer from exposure to ≥8 fiber-years. Using the consensus report estimates (7), it has been suggested that a lower bound of asbestos exposure for screening should be 20–25 fiber-years. However, none of these estimates seem to adequately account for tobacco smoking. In their highly asbestos-exposed group, Gustavsson et al (9) computed a RR of lung cancer of 10 and 43 among non- and current smokers, respectively. Thus in determining eligibility for LDCT screening among asbestos-exposed workers, estimates of RR for lung cancer in relation to both smoking and asbestos exposure must be used.
It has now been generally accepted on the basis of the NLST (1) that an individual who has accumulated 30 pack-years of cigarette smoking becomes eligible for LDCT screening once they reach 55 years. If they have stopped smoking, cessation should be ≤15 years ago. As the only evidence of efficacy of LDCT screening in reducing mortality from lung cancer currently comes from the NLST, we must use the NLST eligibility criteria as a basis for developing recommendations on screening asbestos-exposed individuals.
If someone started smoking at age 15 and smoked a pack a day continuously – and did not stop – the NLST criterion would have been exceeded by the time the individual reached age 55; even if smoking had ceased at age 45, the person would still be eligible for LDCT screening. Thus continuing smokers who smoke ≥1 pack/day would be eligible for LDCT screening at all ages from 55–74, irrespective of their level of asbestos exposure.
A half a pack/day continuing smoker would not accumulate 30 pack-years until he reached the age of 75, so, according to the 30 pack-year and age NLST criteria, he would never be eligible for LDCT screening. However, we should note the dominant effect of duration of smoking on lung cancer risk (10). Furthermore, ex-smokers retain the risk they acquired by the time they stop smoking. Nevertheless, if a smoker stops at age 40, the risk of lung cancer will be low.
Given the above, to justify LDCT screening, exposure to asbestos would only need to be considered for light smokers (<1 pack/day), never smokers, or those who stopped smoking >10 years ago.
To provide further guidance on eligibility for LDCT screening among asbestos-exposed individuals, we have incorporated an estimate of risk for asbestos exposure in the CRMM-LC version 2.1. (Available on the Cancer View Canada website: cancerview.ca/cancerriskmanagement.)
Methods
CRMM-LC employs Canadian demographic and health data, health utilities, standard disease-specific diagnostic and treatment practices, healthcare costs, expected personal income, and tax revenue, and performs simulations at the individual level over a lifespan (4, 5). CRMM-LC incorporates data from the Canadian Cancer Registry (CCR) database, census data, medical literature, and, where data is insufficient, expert opinion. CRMM-LC allows analysis by specific age groups, smoking rates, and screening frequency, and is able to produce outputs reflecting changes in life-years, costs, and specific resource utilization. Costs are expressed in 2008 Canadian dollars, (1 euro = $CAD 1.5). Costs and health adjusted life-years (defined as in reference 4) are discounted at a 3% annual rate. In the present paper, in accordance with current usage, we use the term quality-adjusted life years (QALY) instead of health-adjusted life-years. Further details on the derivation and working of the model are provided in the appendix (http://www.sjweh.fi/data_repository.php).
We considered whether the cost estimates might have to be adjusted for a likely higher prevalence of abnormalities among asbestos-exposed versus non-exposed workers as some of the subjects will have radiological signs of asbestosis. Thus Callol et al (11) found suspicious lung lesions among 21% of asbestos-exposed workers during their baseline LDCT screening, but only in 2.2% during their second round of screening (presumably because the investigations after the first screening determined that the lesions identified did not justify further evaluation). On the basis of this observation, we have assumed that the prevalence of abnormalities will be similar to those identified in the NLST and have not made further adjustments.
In CRMM-LC, persons developing lung cancer are assigned a diagnosis of non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) as well as a clinical stage based on the distribution found in the CCR by age and sex. Survival times for persons with lung cancer are derived from published data and applied based on the disease stage and appropriate treatment pathway. Individuals in the model have a competing overall mortality hazard based on the average risks for the whole population according to age, sex, and year of birth, adjusted by smoking status.
In CRMM-LC, a designated preclinical cancer phase was created for an individual based on an exponential distribution for the time of clinical presentation. The mean of the distribution (1.9 years) was informed by the increase in detected cancers by LDCT screening in the NLST data. Sensitivity and specificity of LDCT were estimated based on NLST findings. True or false positive results led to further investigations (and costs) undertaken in proportion to those found in the NLST trial.
Individuals are assigned a diagnosis of NSCLC or SCLC in proportion to CCR data, but only those diagnosed with NSCLC are stage-shifted towards earlier disease to a degree similar to NLST data. Stage-shifting was specific to the round of screening (per NLST) and differed by whether cancer was screen-detected, occurred between screening (interval cancer), or occurred during the early post-screening period when a clinical diagnosis of lung cancer was made. Individuals with lung cancer undertook treatment, incurred costs, and took on the survival hazard appropriate to their stage. To achieve the mortality reduction seen in the NLST, individuals diagnosed with NSCLC through screening were subjected to a stage-specific mortality lead time (during which they would not die of lung cancer) as well as a lower stage-specific mortality hazard ratio. The simulations do not account for any radiation exposure risk from repeated LDCT scans.
It is accepted that some proportion of screened individuals will suffer from over-diagnosis. We derived estimates from the NLST using additional follow-up data obtained by request of NCI and set these estimates at 18% over three annual screens (10% on first screen, 4% on each subsequent screen). Individuals assigned to the over-diagnosed category retained the survival rate of the general population. NLST investigators have published estimates on over-diagnosis very similar to our own (12).
Costs related to screening included the LDCT scans, patient visits to physicians related to screening and any follow-up, and the costs of non-invasive and invasive testing. Investigations resulting from a positive screening LDCT were assumed to occur with the same frequency as undertaken in the NLST.
The control group for analysis was the unscreened Canadian population, and all costs and health-adjusted life measures are stated in relation to this control group. The baseline screening scenario analyzed annual screening for persons in the 55–74 age group. For the biennial scenarios, sensitivity, specificity, and stage-shifting beyond the first screening scan could not be directly derived from NLST. However, Pastorino et al (13) published some data on biennial screening, and we derived biennial screening sensitivity and specificity rates from NLST by assuming that (i) the screening sensitivity and specificity values were as in the NLST and (ii) the interval rates were as in the two-year period after cessation of screening in NLST. Stage-shifting was applied through sensitivity analysis using various values from the NSLT and the unscreened Canadian population.
In modeling the effect of LDCT screening on individuals exposed to asbestos, an asbestos-exposed cohort was simulated. It was initially assumed that these individuals had acquired a RR of 2 for lung cancer in comparison to non-asbestos-exposed individuals. As it has been concluded using CRMM-LC that biennial LDCT screening is more cost-effective than annual screening (Goffin J, Flanagan WE, Miller AB, Fitzgerald N, Memon S, Wolfson M et al. An Estimate of the Cost Effectiveness of Lung Cancer Screening in Canada. Submitted for publication, pending review), biennially screened non-asbestos-exposed individuals – who had the NLST eligibility criteria – were used as the basic comparison for the asbestos-exposed population. CRMM-LC does not include an asbestos-exposure rate explicitly, but a 3-year probability of developing lung cancer can be derived from different risk profiles. It was found that an individual with a 12.5 pack-year smoking history and asbestos exposure resulting in a RR for lung cancer of 2 had the same risk of developing lung cancer as an individual with a 30 pack-year smoking history without asbestos exposure.
Results
The baseline modeled cost of treatment for lung cancer for 2014–2034 with no screening in Canada is CAD$8,878.9 M. The modeled screening scenarios with or without asbestos exposure are summarized in table 1 together with the modeled total costs of screening and treatment, health-related QALY gained, and the resulting incremental cost-effectiveness ratios (ICER) as compared to no screening. All the scenarios modeled for an asbestos-exposed cohort were biennial. If resources and the finances available are such that a decision is taken to offer biennial screening, two of the scenarios modeled for the asbestos-exposed 55–74 year age group are less cost-effective: those with a smoking history of only 10 and 13 pack-years. Thus for individuals with a two-fold risk of asbestos-induced lung cancer to be eligible for LDCT screening, a smoking history of ≥15 pack-years would be necessary. Restricting the age range to individuals 65–74 years of age would not be cost-effective if they had only a 10 pack-year history of smoking. It also would not be cost-effective to screen non-smokers with a history of this degree of asbestos exposure. However, when we model non-smokers with asbestos exposure resulting in a RR for lung cancer of 5 and 10 (the heavily exposed), we find it cost-effective to screen the latter but not the former.
Table 1
Modeled low-dose computerized tomography (LDCT) screening scenarios with summary of output from the scenarios. [QALY= quality-adjusted life years: CAD$=Canadian dollars.]
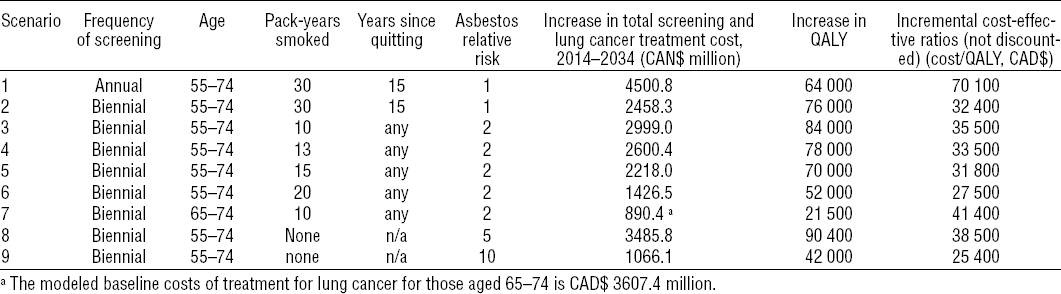
The modeled scenarios for the time period 2012–2032 indicate that the benefits gained by screening decrease with time. This reflects the fact that, in technically advanced countries, asbestos exposure should be declining.
Discussion
CRMM-LC is a computer-based microsimulation model that assembles and synthesizes results from a range of specific analyses, including published studies, using statistical methods. Such models are being increasingly used to facilitate the development of cancer control policies (14–16). Assumptions underlying the model are made explicit, and sensitivity analyses can be conducted allowing estimation of the likely effect of varying these assumptions. The validity of the results cannot be directly assessed, though, as with all decisions taken on public health policy, eventually data will be obtained to enable them to be evaluated.
A major assumption in the results presented here is that the benefit from lung cancer screening demonstrated in the NLST screening trial can be replicated in a general population, though there are indications from preliminary results from one of the European lung screening trials (13) that the level of efficacy seen in the NLST may not be readily replicated. Other assumptions are that screening for lung cancer in Canada will be introduced through organized provincial programs that reflect current best practice, that asbestos-exposed workers will be assessed for screening in such programs, and that the prevalence of false positives and negatives will not be higher than those that occurred in NLST.
One potential harm from screening that is difficult to model is the anxiety that may be induced by the detection of benign abnormalities as well as false positives. Although in most screening programs for other cancers these concerns have been demonstrated to be short-lived, among asbestos-exposed workers pulmonary abnormalities including pleural plaques may be detected. A study in France documented increased stress in asbestos-exposed subjects screened by LDCT with such abnormalities detected that persisted to ≥6 months (17). Increased care may therefore be needed in asbestos-exposed workers found to have such abnormalities assuring them of the benign nature of the lesions.
One challenge in modeling LDCT screening among asbestos-exposed workers is the interaction between asbestos exposure and smoking in increasing the risk of lung cancer. There have been several attempts to assess this, but in general, it has been concluded that the interaction is multiplicative (18). In our modeling, we have implicitly assumed that the interaction is multiplicative, inherent in the relative risk functions we have applied. However, even if the interaction is sub-multiplicative, the relative risk for asbestos-exposed workers who are currently smoking or are former smokers which we have applied would be very similar to that recently reported by Markowitz et al (19) for asbestos-exposed non-smoking insulators. Given the uncertainty in the estimation of extent of prior asbestos exposure, it is unlikely that uncertainty in the application of the degree of interaction between the two exposures will have a major effect.
Given the fact that LDCT screening is not free from harm – both from false positive investigations and from overdiagnosis of lung cancer – we believe that it is important to attempt to quantify the degree of risk of lung cancer for an asbestos-exposed worker, rather than simply labeling all workers previously exposed to asbestos as at sufficiently high risk to justify LDCT screening. Thus those who advocate LDCT screening for asbestos-exposed workers will have to accept the responsibility for estimating the degree of that exposure to justify any recommendation they may make for such screening. However, we concede that computing future risk of lung cancer is an uncertain endeavor, and we encourage full documentation and evaluation of all attempts to do so, so that eventually recommendations for LDCT screening among asbestos-exposed workers are based on a more secure foundation.
Our estimates of the cost-effectiveness of LDCT screening are generally lower than those previously published based on US data, largely because they were based on annual screening, and screening was assumed to be applied to heavy smokers aged 50–70 years (14). In contrast, our estimates are based upon biennial screening commencing at age 55.
Concluding remarks
Individuals who have accumulated 30 pack-years of cigarette smoking are eligible for LDCT screening once they have reached the age of 55, irrespective of their degree of asbestos exposure. If they have stopped smoking, cessation should be ≤15 years ago.
Continuing smokers who smoke ≥1 pack a day, irrespective of their degree of asbestos exposure, are eligible for LDCT screening at all ages from 55–74, providing they commenced smoking no later than age 25.
Asbestos-exposed individuals with an estimated two-fold or more risk of lung cancer from asbestos-exposure are eligible for LDCT screening at all ages from 55–74 if they have a history of 15 pack-years or more of cigarette smoking.
Asbestos-exposed individuals who are lifelong non-smokers are eligible for LDCT screening at all ages from 55–74 if they have accumulated a degree of asbestos exposure resulting in an estimated risk of lung cancer of ≥10.



