Sedentary lifestyle, or physical inactivity, is an established risk factor for cardiovascular disease (1–3). Accordingly, physical activity (PA) both at work and during leisure time has been recommended (4). While leisure-time PA (LTPA) has been well documented to promote health (5, 6), the effect of occupational PA (OPA) is inconsistent (7). Without adjustment for LTPA, higher levels of OPA were reported to: (i) be protective against cardiovascular disease (CVD) (8, 9), (ii) have no effect (10, 11), or (iii) increase the CVD risk (12, 13). When adjusting for LTPA, some studies showed that greater OPA is associated with progression of carotid atherosclerosis (14) and increased AMI incidence (15) or risk of IHD mortality (16).
One explanation for such inconsistencies could be that the effects of OPA depend on the level of LTPA (17, 18) and possibly individual aerobic fitness (14, 19). If interaction between OPA and LTPA exists, a model ignoring such interaction could, in some scenarios, result in cancellation of the effect of one variable across levels of another and yield a misleading average estimate of no effect. Another explanation could be that high and exhausting levels of OPA preclude workers from engaging in LTPA (20); thus, these workers cannot benefit from LTPA. Finally, OPA could directly affect CVD (21). Based on the negative correlation between OPA and LTPA observed in previous work (14) and previous observations that LTPA participation was relatively low among blue-collar workers (22), we hypothesized that LTPA would both interact with OPA and mediate the effect of OPA on CVD. Therefore, following up on our previous work examining the relation between OPA and cardiovascular outcomes (14, 23), we further assessed the modifying and mediating roles of LTPA on the pathway from OPA to 20-year incidence of AMI. We also explored the impact of LTPA on AMI at different levels of OPA. We conducted separate analyses for men with and without preexisting ischemic heart disease (IHD), as past studies suggested a heterogeneous OPA effect by IHD status (15).
More specifically, and separately for men with and without preexisting IHD at baseline, our study aimed to: (i) assess both multiplicative and additive interaction between OPA and LTPA and their combined effect on 20-year incidence of AMI and (ii) examine the potential mediating role of LTPA on the pathway from OPA to AMI, using causal mediation analysis that allowed for exposure–mediator interaction (24, 25).
Methods
Study design, setting and population
Participants were from the prospective Kuopio Ischemic Heart Disease Risk Factor (KIHD) Study, an age-stratified, random, population-based sample of Eastern Finnish men, residing in the city of Kuopio or its surrounding rural communities. Details of the study population are available elsewhere (15, 26). Out of 3235 eligible men aged 42, 48, 54, or 60 years at baseline, 2682 (82.9%) men agreed to participate, with 553 men being excluded due to refusal (N=367) and no contact (N=186). All participants underwent baseline examinations and interviews between March 1984 and December 1989 and were passively followed by national hospitalization discharge and death registries until 2011. We excluded 791 participants who were not working at baseline or in the 12 months prior, resulting in a final study sample of 1891 participants with complete information on all the baseline covariates for the main analyses. All participants provided written informed consent. The University of Eastern Finland (former University of Kuopio) Research Ethics Committee and the University of California, Los Angeles, (UCLA) Institutional Review Board approved this study.
Assessment of incidence of acute myocardial infarction
As described previously (15), we ascertained first-time incident AMI (ICD-9 code 410) during follow-up via record linkage with national hospital discharge and death registries including the national AMI register established under the World Health Organization’s “Monitoring of Trends and Determinants of Cardiovascular Diseases (MONICA)” project (27, 28). A university-based cardiologist for this study confirmed hospital discharge diagnoses using other hospital records, lab results, and electrocardiograms. We censored the follow-up at 31 December 2011 or date of death, whichever came first.
Assessment of occupational physical activity
We measured OPA as relative aerobic strain (RAS), the most predictive measurement for AMI among other OPA measures examined in the same study population (23). RAS (or relative aerobic workload) expresses the caloric demands of work as a percentage of the individual worker’s aerobic cardiorespiratory fitness or maximal work capacity (%VO2max) (29). RAS takes into account both the absolute energy expenditure (EE) and the workers’ individual aerobic capacity. Detailed descriptions of the assessment of these variables can be found elsewhere (23, 29). Based on work physiology and ergonomic principles, it is often the misfit between high job-related energy demands and low worker aerobic capacity, rather than a high absolute amount of EE alone, that will lead to elevated blood pressure and heart rate, two established risk factors for AMI (23, 30). Also, OPA has been shown to be detrimental to workers with low cardiorespiratory fitness but not to those with high fitness level (31). Thus, we decided to use RAS as our OPA exposure measure in the main analyses.
Absolute EE at work (in kcal/day) was assessed from baseline interview data on time spent in various activities at work during a typical workday and reference data on the energy requirements (kcal/kg/hour) of these activities. EE in kcal for each reported activity was calculated by multiplying the duration (hours per day) by the respective intensity (MET) and body weight (kg) for each individual. EE per typical workday was the sum of EE for all activities.
Cardiorespiratory fitness (also known as aerobic capacity or VO2max) was measured with a maximal, symptom-limited exercise-tolerance test on a bicycle ergometer as explained in detail elsewhere (32–34). VO2max, in ml O2 per kg per minute, was defined as the highest value or plateau in oxygen uptake during maximal symptom-limited bicycle ergometer and was standardized by body weight.
To avoid confusion in terminology, we used RAS or absolute EE when describing results from specific analyses or studies and used OPA as a general term for the entire OPA domain to facilitate discussion.
Assessment of leisure-time physical activity
LTPA was measured using the KIHD 12-month LTPA history, a modified version of the Minnesota LTPA questionnaire (35), which included the 16 most common LTPA of middle-aged Finnish men (34, 36). Respondents were asked to record the frequency, duration, and intensity of each of 16 activities performed for each of the 12 previous months. Conditioning (vigorous intensity) LTPA included walking, jogging, cross-country skiing, bicycling, swimming, rowing, ball games, gymnastics, dancing, or weightlifting. We calculated the sum of these activities and obtained the average-conditioning LTPA, expressed in minutes per week, in the previous year. Unless otherwise noted, we used LTPA to represent conditioning LTPA throughout the article.
Assessment of covariates
We included as confounders age, education, participation in an unrelated lipid-lowering drug trial (placebo group, treatment group, versus none), and baseline IHD. Education was categorized into: (i) some elementary school, (ii) elementary school completed or elementary school plus some junior high school, (iii) junior high school completed or junior high school plus some senior high school, and (iv) senior high school completed or beyond. A continuous smoking variable “pack-years” was calculated as the number of packs (20 cigarettes/pack) per day times the number of years smoked. Alcohol consumption (grams per week) accounted for the frequency of drinking and amount of drinks per occasion for each type of alcoholic beverage (beer, wine, spirits) for the last 12 months. Psychosocial job factors were measured using questionnaires that captured mental strain at work (11 items of psychological demands), social support at work (3 items), and stress from work deadlines. These factors have been associated with progression of atherosclerosis and an increased risk for myocardial infarction and mortality in this study population and showed satisfactory Cronbach’s α coefficients (37, 38). We classified participants as having preexisting IHD at baseline if they (i) had a history of prior (before baseline) myocardial infarction or angina pectoris, (ii) currently used anti-angina medication, or (iii) had positive findings of angina according to the London School of Hygiene cardiovascular questionnaire (39).
Interaction analysis
We used Cox proportional hazard models (40) with adjustment for covariates listed in table 1. We added an OPA × LTPA product term to assess the interaction between OPA and LTPA on the multiplicative scale. We also calculated the relative excess risk for interaction (RERI) as a measure of additive interaction (41): HR11 – HR10 – HR01 + 1, where HR11, HR10, and HR01 respectively represented the joint effect of OPA and LTPA, the main effect of OPA, and the main effect of LTPA. A positive value for RERI (ie, >0) would indicate that the combined effect of OPA and LTPA is greater than the sum of their separate effects assuming monotonic effects of both exposures (42). Variables were recoded jointly when necessary so that the reference combined category represented the lowest risk group (43). OPA was modeled as a binary indicator (RAS >33% as high versus RAS ≤33% as low), based on the maximum level of 33% VO2max recommended for 8 hours of work (29, 44). Similarly, LTPA was modeled as a binary indicator (LTPA ≥75 minutes/week as high versus LTPA <75 minutes/week as low) based on WHO global recommendations (45). We also performed the analyses using low OPA and high LTPA as the reference category for binary PA measures and compared different combinations of OPA and LTPA relative to this reference group as done in the existing literature. We reported the hazard ratio (HR) for AMI associated with a 1-unit increase in OPA and its corresponding 95% confidence interval (CI) at different levels of LTPA and vice versa.
Table 1
Characteristics of the study sample and distribution of exposure and covariates by preexisting ischemic heart disease (IHD) status, Kuopio Ischemic Heart Disease Risk Factor Study, 1984–2011 (N=1891). [SD=standard deviation.]
| Men without IHD (N = 1565) | Men with IHD (N = 326) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Mean | SD | N | % | Mean | SD | N | % | |
| Occupational physical activity (OPA) | ||||||||
| Relative aerobic strain (%) | 29.7 | 12.1 | 38.5 | 16.4 | ||||
| Binary relative aerobic strain (>33%) | 490 | 31.3 | 179 | 54.9 | ||||
| Absolute energy expenditure (kcal/day) | 2078 | 875 | 2272 | 970 | ||||
| Leisure-time physical activity (LTPA) | ||||||||
| Conditioning LTPA (minutes/week) | 105 | 117 | 109 | 145 | ||||
| Binary conditioning LTPA (≥75 minutes/week) | 742 | 47.4 | 143 | 43.9 | ||||
| Cardiorespiratory fitness (VO2max, O2/kg/minute) | 32.8 | 7.0 | 27.5 | 6.9 | ||||
| Age at baseline (years) | 51.5 | 5.1 | 53.5 | 3.9 | ||||
| 42 | 292 | 18.7 | 17 | 5.2 | ||||
| 48 | 274 | 17.5 | 47 | 14.4 | ||||
| 54 | 916 | 58.5 | 233 | 71.5 | ||||
| 60 | 83 | 5.3 | 29 | 8.9 | ||||
| Participation in lipid-lowering drug trial | ||||||||
| Placebo group | 135 | 8.6 | 28 | 8.6 | ||||
| Treatment group | 136 | 8.7 | 27 | 8.3 | ||||
| Education | ||||||||
| Some elementary school | 113 | 7.2 | 41 | 12.6 | ||||
| Elementary school completed/some junior high school | 1144 | 73.1 | 251 | 77.0 | ||||
| Junior high school completed/some senior high school | 150 | 9.6 | 28 | 8.6 | ||||
| Senior high school completed or beyond | 158 | 10.1 | 6 | 1.8 | ||||
| Behavioral factors | ||||||||
| Smoking (pack-years) | 7.1 | 14.5 | 10.5 | 17.4 | ||||
| Alcohol consumption (g/week) | 71.5 | 111.8 | 88.3 | 196.4 | ||||
| Psychosocial job factors | ||||||||
| Mental strain at work index a | 11.5 | 6.3 | 13.4 | 7.1 | ||||
| Social support at work score b | 6.5 | 2.5 | 6.5 | 2.4 | ||||
| Stress from work deadlines | 357 | 22.8 | 105 | 32.2 | ||||
Mediation analysis
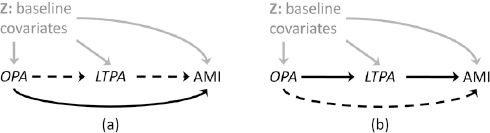
We assumed that baseline OPA level determined the baseline LTPA level based on the negative correlation observed between OPA and LTPA in the same cohort (14) and past literature (20, 22) and that first-time incidence of AMI occurring during follow-up can be attributed to the OPA and LTPA levels measured at baseline. We invoked the stable unit treatment value assumption (46) and assumptions of consistency, positivity, conditional exchangeability (no-uncontrolled confounding) (47, 48), and no selection bias and measurement error. Further discussions of these assumptions can be found elsewhere (49). We used recently proposed inverse-probability weighted fitting of marginal structural models for causal mediation analysis (24) to estimate the marginal pure direct effect of baseline OPA on AMI and the marginal total indirect effect of baseline OPA via baseline LTPA (Figure 1). Pure direct effect was defined as the HR comparing high to low OPA levels while allowing LTPA to attain the natural value under the low OPA level. Total indirect effect was defined as the HR comparing two LTPA levels – the natural LTPA level under high OPA versus the natural LTPA level under low OPA – while setting OPA level to be high. Methodological details are in the appendix (www.sjweh.fi/index.php?page=data-repository).
Sensitivity analysis
We conducted the following sensitivity analyses to check the robustness of our results for assessing interaction between OPA and LTPA. First, we repeated our analyses using both continuous and trichotomized measures for OPA and LTPA. Details of these measures are in the appendix. Second, we repeated our main analyses with additional adjustment for biological factors including blood glucose, plasma fibrinogen, body mass index, LDL- and HDL-cholesterol, systolic blood pressure, lipid-lowering medication, and anti-hypertensive medication. Third, we used continuous absolute EE (500 kcal increase), centered at the population mean of 2111 kcal/day, as an alternative measure of OPA to assess the interaction between OPA and LTPA.
For assessing the mediating role of LTPA, we additionally included 4-year LTPA as a second mediator and examined the effect of OPA via pathways involving baseline LTPA, 4-year LTPA, or neither. We further restricted our analytical sample to 455 men without baseline IHD and who had complete information on all variables. Detailed sample restriction criteria, methodology, effect definition, and implementation steps can be found elsewhere (25) and in the appendix.
All analyses were performed using Stata, version 14 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the study sample
The distribution of exposure variables and covariates by preexisting IHD status is listed in table 1. Participants’ mean age at baseline was 51.5 [standard deviation (SD) 5.0] years for participants without IHD and 53.5 (SD 3.9) years for those with IHD. Over 70% of the participants completed elementary school but not junior high school. Participants with IHD had higher levels of RAS, absolute EE, and mental strain at work, smoked and drank more, and experienced more stress from work deadlines, and had a lower level of fitness than men without IHD. LTPA and social support at work were similar in these two subgroups.
Incidence of AMI
During an average of 19.56 (SD 7.53, range 0.01–27.76) years of follow-up and a total person-time of 36 991 years, 495 first-time (after baseline) incident AMI occurred among 1891 study participants, yielding a yearly incidence rate of 1.34%. Among 1565 men without baseline IHD, 353 AMI occurred (yearly incidence 1.11%) whereas among 326 men with baseline IHD, 142 AMI occurred (yearly incidence 2.60%).
Interaction between binary OPA and binary LTPA in affecting AMI
Table 2 displays the associations between one PA domain and AMI at different levels of the other PA domain by preexisting IHD status and the joint association of both OPA and LTPA with AMI, using the combination of low OPA and high LTPA as the reference. For men without IHD, high OPA (RAS >33%) was only positively associated with AMI incidence among men with low LTPA and with age adjustment (HR 1.34, 95% CI 1.01–1.76) but this association attenuated after adjusting for other factors (HR 1.27, 95% CI 0.96–1.68). For men with IHD, high OPA positively predicted AMI at low LTPA (HR 1.59, 95% CI 0.99–2.57) but not at high LTPA (HR 1.04, 95% CI 0.61–1.79). For both IHD subgroups, high LTPA was weakly negatively associated with AMI at high but not at low OPA level. Compared to men with low OPA and high LTPA, men with high OPA and low LTPA had the highest risk for AMI, irrespective of IHD status (HR 1.33, 95% CI 0.99–1.78 for men without IHD, HR 1.36, 95% CI 0.84–2.18 for men with IHD). Multiplicative interaction between OPA and LTPA was observed among men with IHD (ratio of HR 0.65, P=0.240) but no additive interaction was observed for both subgroups.
Table 2
Hazard ratios (HR) and 95% confidence intervals (95% CI) for the main effect and joint effect of occupational physical activity (OPA) and leisure-time physical activity (LTPA) on 20-year incidence of acute myocardial infarction (N=495) when both domains of physical activity were modeled as binary variables,a by preexisting ischemic heart disease (IHD) status, Kuopio Ischemic Heart Disease Risk Factor Study, 1984–2011 (N=1891). [95% CI=95% confidence interval; RERI=relative excess risk for interaction.]
| Men without IHD (N=1565) | Men with IHD (N=326) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Age-adjusted | Fully-adjusted b | Age-adjusted | Fully-adjusted b | ||||||||
| N | HR | 95% CI | HR | 95% CI | N | HR | 95% CI | HR | 95% CI | ||
| LTPA level | OPA level | ||||||||||
| Low | Low | Reference | Reference | Reference | Reference | ||||||
| High | 1.34 | 1.01–1.76 | 1.27 | 0.96–1.68 | 1.52 | 0.95–2.42 | 1.59 | 0.99–2.57 | |||
| High | Low | Reference | Reference | Reference | Reference | ||||||
| High | 1.20 | 0.83–1.74 | 1.10 | 0.76–1.60 | 0.97 | 0.58–1.63 | 1.04 | 0.61–1.79 | |||
| OPA level | LTPA level | ||||||||||
| Low | Low | Reference | Reference | Reference | Reference | ||||||
| High | 0.85 | 0.66–1.11 | 0.95 | 0.73–1.25 | 1.18 | 0.70–1.99 | 1.17 | 0.69–2.01 | |||
| High | Low | Reference | Reference | Reference | Reference | ||||||
| High | 0.77 | 0.53–1.12 | 0.83 | 0.57–1.21 | 0.76 | 0.48–1.19 | 0.77 | 0.48–1.23 | |||
| P-value for multiplicative interaction c | 0.649 | 0.543 | 0.205 | 0.240 | |||||||
| Combinations of OPA and LTPA, using the lowest risk group as reference | |||||||||||
| Low OPA/high LTPA | 571 | Reference | Reference | 80 | Reference | Reference | |||||
| High OPA/high LTPA | 171 | 1.20 | 0.83–1.74 | 1.10 | 0.76–1.60 | 63 | 0.97 | 0.58–1.63 | 1.04 | 0.61–1.79 | |
| Low OPA/low LTPA | 504 | 1.17 | 0.90–1.52 | 1.05 | 0.80–1.37 | 67 | 0.84 | 0.50–1.42 | 0.85 | 0.50–1.46 | |
| High OPA/low LTPA | 319 | 1.57 | 1.19–2.07 | 1.33 | 0.99–1.78 | 116 | 1.28 | 0.83–1.99 | 1.36 | 0.84–2.18 | |
| RERI d | 0.19 | -0.37–0.75 | 0.18 | -0.32–0.69 | -0.55 | -1.49–0.38 | -0.54 | -1.51–0.43 | |||
a Low OPA: relative aerobic strain (RAS) ≤33%; high OPA: RAS >33%; low LTPA: <75 minutes/week; high LTPA: ≥75 minutes/week.
b Model adjusted for age, education, participation in an unrelated clinical trial, smoking, alcohol consumption, mental strain at work, social support at work, and stress from work deadlines.
d RERI were measures for additive interaction. For men without IHD, RERI were calculated as HRhigh OPA, low LTPA – HRhigh OPA, high LTPA – HRlow OPA, low LTPA +1. For men with IHD, RERI were calculated as HRhigh OPA, high LTPA – HRhigh OPA, low LTPA – HRlow OPA, high LTPA + 1, using low OPA and low LTPA as the reference group.
Results from sensitivity analysis using continuous PA measures are presented in appendix table A1 (www.sjweh.fi/index.php?page=data-repository). For men without IHD, higher OPA level—a 20% increase in RAS from a reference level of 23.5%—positively predicted AMI incidence at both LTPA of 0 and 75 minutes/week (HR 1.45, 95% CI 1.19–1.75, and HR 1.49, 95% CI 1.28–1.75, respectively. Weaker but still substantial positive associations between OPA and AMI were found among men with IHD (HR 1.25, 95% CI 0.96–1.64 at LTPA of 0 minutes/week; HR 1.32, 95% CI 1.07–1.62 at LTPA of 75 minutes/week). We found no association between LTPA and AMI across levels of OPA and no multiplicative interaction between continuous OPA and LTPA. Detailed descriptions for results from sensitivity analyses that (i) used trichotomized PA measures, (ii) additionally adjusted for biological factors, and (iii) used absolute EE as OPA measure are presented in the appendix (tables A2a – A4) (www.sjweh.fi/index.php?page=data-repository).
Mediating role of LTPA on pathway from OPA to AMI
Table 3 depicts the pure direct effect of OPA on AMI and the total indirect effect via baseline LTPA, estimated using inverse-probability weighted fitting of marginal structural models. We observed similar effect estimates from all methods that differed only in the way confounding was handled. For men without IHD, the estimate for total effect of OPA on AMI was 1.31 (95% CI 0.99–1.79) when marginalizing over all covariates and LTPA. The majority of the positive association of OPA with AMI was attributable to pathways other than through LTPA (pure direct effect 1.27, 95% CI 0.93–1.74). The effect estimates for men with IHD were similar to that among men without IHD.
Table 3
Hazard ratios (HR) and 95% confidence intervals (95% CI) for the pure direct effect (PDE) and total indirect effect (TIE) of occupational physical activity (OPA) as measured by binary relative aerobic strain [with leisure-time physical activity (LTPA) as mediator] on 20-year incidence of acute myocardial infarction (N=495) for all men, stratified by preexisting ischemic heart disease (IHD) status and estimated using inverse-probability weighted (IPW) fitting of marginal structural models (MSM)a, in the Kuopio Ischemic Heart Disease Risk Factor Study, 1984–2011 (N=1891).
| Method | Men without IHD (N=1565) | Men with IHD (N=326) | ||
|---|---|---|---|---|
|
|
|
|||
| HR b | 95% CI | HR b | 95% CI | |
| MSM c | ||||
| PDE | 1.27 | 0.93–1.74 | 1.28 | 0.88–1.90 |
| TIE | 1.04 | 0.88–1.22 | 1.04 | 0.88–1.27 |
| Total effect | 1.31 | 0.99–1.79 | 1.33 | 0.97–1.85 |
| Conditional MSM d | ||||
| PDE | 1.20 | 0.96–1.55 | 1.28 | 0.87–1.93 |
| TIE | 1.02 | 0.90–1.15 | 1.05 | 0.90–1.24 |
| Total effect | 1.22 | 1.02–1.54 | 1.35 | 0.96–1.91 |
| Doubly robust MSM e | ||||
| PDE | 1.23 | 0.93–1.67 | 1.30 | 0.88–1.95 |
| TIE | 1.04 | 0.90–1.21 | 1.04 | 0.89–1.24 |
| Total effect | 1.28 | 0.98–1.70 | 1.34 | 0.97–1.92 |
| Conditional total effect f | 1.22 | 0.97–1.53 | 1.34 | 0.94–1.91 |
a OPA was measured by binary relative aerobic strain (RAS) indicator (RAS >33% versus RAS ≤33%) and LTPA was dichotomized (≥75 minutes/week versus <75 minutes/week). Covariates included age, education, participation in an unrelated clinical trial, smoking, alcohol consumption, mental strain at work, social support at work, and stress from work deadlines.
c IPW was created based on a weight for OPA (dealing with confounding) and a weight for LTPA (decomposing effect)
d IPW was created based on a weight for LTPA (decomposing effect) only. Conditional MSM included covariates to control for confounding.
Table A5 in the appendix depicts results from sensitivity analysis that used also LTPA measured at 4-year follow-up and further decomposed the total effect into baseline LTPA pathway-specific effect, 4-year LTPA pathway-specific effect, and natural direct effect that is through neither baseline nor 4-year LTPA. In this restricted sample of men without IHD, OPA was not associated with AMI overall (total effect 1.08, 95% CI 0.58–2.02). Weak positive natural direct effect (1.22, 95% CI 0.57–2.46) and weak negative indirect effect via baseline LTPA (0.93, 95% CI 0.69–1.21) were observed. Follow-up LTPA at 4 years did not mediate the effect of baseline OPA on AMI (0.96, 95% CI 0.79–1.05).
Discussion
This 20-year follow-up study examined the interaction between OPA and LTPA in affecting AMI incidence and whether LTPA mediated the effect of OPA on AMI among men with and without preexisting IHD. We found that high levels of OPA positively predicted AMI at low LTPA levels for both IHD subgroups. LTPA was not predictive of AMI after accounting for OPA in most combinations, although sensitivity analysis indicated that the effect of LTPA may be non-linear across levels of OPA and by IHD status. We found potential multiplicative but not additive interactions between OPA and LTPA among men with IHD. LTPA did not appear to mediate the effect of OPA on AMI.
Effects of OPA on AMI, accounting for LTPA
Our finding of the positive link between OPA and AMI, regardless of categorical or continuous PA measures being used, is in line with a previous study on OPA and AMI from the same cohort, despite slightly different covariate adjustment (14). Our study confirmed the previous finding that conditional on LTPA, a relative OPA measure (RAS) that accounts for individual cardiorespiratory fitness was more predictive of AMI than an absolute OPA measure (EE) (14, 23). This finding is robust against the choice of OPA measure and modeling schemes. It is consistent with an overall harmful effect of OPA on coronary heart disease (CHD) as recently synthesized based on five prospective cohort studies published in 2011, 2012, and the first quarter of 2013 (21) but not with two other prospective observational studies published earlier that suggest a protective effect (50) or no effect (51). All seven studies investigated the impact of OPA on health outcomes while simultaneously accounting for LTPA: one found a positive association with IHD mortality (16); one found a positive association with CHD incidence only among men with high LTPA levels but not among men with low LTPA levels (17); three found no associations with myocardial infarction (52) or CHD incidence (51, 53); and two found negative associations with IHD incidence when additionally accounting for occupational heavy lifting (54), or with CHD incidence when additionally accounting for commuting PA (9). Adjusted for LTPA, one recent study reported elevated risk for CHD mortality (55). Additionally accounting for physical fitness, Holtermann and colleagues (56) found an increased risk of IHD mortality associated with high OPA in the least and moderately fit group, but not among the most fit men. Similarly, Clays et al (19) reported a positive association between high OPA and mortality and that association was particularly pronounced among workers with low physical fitness. Our study examined OPA effects with a commonly used absolute EE measure but also the preferred measure of RAS at work that took into account individual cardiorespiratory fitness, which, we believe, is crucial in studies examining the effect of different PA domains on cardiovascular health as demonstrated in this paper and our previous report (14). The use of continuous versus broad categorical PA measures, difference in definition and categorization of these measures, and different study endpoints can also be reasons for the inconsistent findings in the current study as well as in the literature (15).
Effects of LTPA, accounting for OPA
Based on our analyses using dichotomized or continuous measures for PA, our study did not confirm the accumulated evidence on and the long-held belief in the cardioprotective effect of LTPA, at least not among working middle-aged men (21, 57). Based on our sensitivity analysis of trichotomized PA measures, mixed results were found for the impact of high levels of LTPA: it appeared to decrease AMI risk for men at low OPA and with IHD, increase AMI risk for men at moderate OPA (regardless of IHD status, and up to 2.58–fold, 95% CI 1.03–6.45 among men with IHD), or have no effect on AMI at high OPA level. In fact, the evidence from the few previous studies that account for OPA is not consistent either: for workers with high OPA, high LTPA was found to be preventive in some studies (16, 52) but harmful in others (17). A recent cluster randomized controlled trial also reported opposing health impacts of a 4-month aerobic exercise intervention among Danish cleaners. While the intervention increased cardiorespiratory fitness, lowered resting and sleeping heart rate, reduced inflammation markers, and reduced relative workload (measured as percent heart rate reserve, which is equivalent to RAS), it also significantly increased systolic blood pressure (58). These mixed findings cast doubt on whether the international PA recommendation for the public at large (45) is similarly applicable to working populations with high physical job demands or with preexisting CVD.
Biological plausibility: OPA and LTPA elicit different physiological responses
OPA and LTPA have different inherent characteristics. Mandatory OPA often has high frequency and long duration, involves activities such as heavy lifting, bending, pushing and pulling, monotonous and static postures with limited ability for pauses and restitution, and may not allow for adequate rest periods (15, 59). Such long-term high cardiovascular workloads can cause atherosclerosis via a prolonged elevated heart rate that leads to increased intravascular turbulence, unfavorable wall shear stress, endothelial injury (60–63), and in turn inflammatory processes in the arterial walls (14), according to the hemodynamic-inflammatory theory of atherosclerosis (63). Another proposed physiological mechanism involves high OPA-induced elevation in systolic blood pressure over the day (during work, at home, and during sleep) (64), which is a strong predictor for cardiovascular events (65). In contrast, voluntary LTPA is usually of shorter duration compared to OPA, involves more dynamic movements, and is characterized by sufficient variation and time for restitution (59). Thus, people engaging in LTPA can achieve a training effect on the heart by performing relatively few and short but intensive bursts of momentarily exhausting conditioning PA in leisure (7). However, such activities are typically not performed during long hours of physically demanding work nor—due to exhaustion—after work. A recent study among Danish cleaners found that they spent most of their work being on their feet, and on average worked 28% of the time exceeding recommended maximum levels of RAS during work (>30%) that will lead to excessive exhaustion, but virtually never reached levels of RAS (>60%) that would confer any training benefit during or after work. After work, these cleaners spent most of their time sitting or lying down in order to recuperate from their exhausting work (20).
Combined effects of OPA and LTPA
When assessing the combined effect of both OPA and LTPA, we found men with high OPA and low LTPA had the highest risk for AMI. This is in line with the above theory on the different health impact of OPA versus LTPA, and the Belgian Physical Fitness Study (19). However, using similar cutoff points to divide low versus moderate or high LTPA for men without preexisting IHD, our result did not support the finding by another Belgian study that showed an almost four times increased incidence of coronary events comparing men with high OPA and high LTPA to men with low OPA and high LTPA (17). Instead, we found that men with high OPA and low LTPA had the highest risk for AMI (HR 1.36, 95% CI 0.98–1.89), which is consistent with our main result. A recent study in Israel found that employees who performed moderate-to-hard OPA (self-reported) and no LTPA had the greatest risk for all-cause mortality (55). The combination of low OPA and low LTPA, averaging over levels of commuting PA, was associated with the highest risk for heart failure among Finnish men (66). Given these mixed findings in the literature, the question of whether workers with high OPA would benefit from being highly physically active during leisure time (59), remains open. Aside from this uncertainty, the need for rest of workers in physically demanding work may prevent them from engaging in such LTPA regardless. In fact, our study showed that high OPA (RAS) at baseline predicted lower LTPA during 4-year follow-up [adjusted odds ratio (ORadj) 0.56, 95% CI 0.44–0.70 among men without IHD; ORadj 0.51, 95% CI 0.32–0.83 among men with IHD]. Therefore, it may be necessary, instead, to lower physical work demands for these workers, allow for longer rest periods, or redesign work tasks so that exhausting effects are minimized and some training effects included.
Sensitivity analyses and implications for future studies
By comparing results from main and sensitivity analyses, we found patterns of the effects of PA domains on AMI that may be worth considering in future studies. First, the impact of OPA on AMI within a specific level of LTPA appeared non-linear. Based on our analysis using continuous PA measures, quadratic terms for OPA measures may not capture such non-linearity (results not shown). Second, how OPA is related to AMI (ie, the shape of the relation) may differ across different levels of LTPA. These two points also apply to the relation between LTPA and AMI by levels of OPA. Third, results from analyses that use dichotomized PA measures may also depend on the cut-off points chosen for the categorization. To further complicate matters, the pattern seems to differ by preexisting IHD status as well.
OPA and LTPA among workers with preexisting CVD
Few studies examined the interplay between OPA, LTPA, and fitness on cardiovascular outcomes among workers with preexisting CVD. One study among Copenhagen men with preexisting CVD (67) found no association between moderate or high OPA and IHD mortality and a positive but uncertain association between high OPA and all-cause mortality. We observed a greater impact of OPA (RAS) on AMI at low compared to high LTPA levels among men with IHD. Importantly, both studies failed to find negative associations between LTPA and cardiovascular outcomes. Compared to their counterparts free of IHD at baseline, these men had higher OPA levels (in terms of both absolute EE and relative aerobic strain) but lower levels of cardiorespiratory fitness. They may be more likely to experience an overloading associated with job-related heavy and especially static work on their cardiovascular system (68) and thus experienced a more detrimental health impact of high OPA than men without preexisting CVD as well as no benefit from LTPA. The uncertainty of the positive association between OPA and AMI could be attributed to the small sample size. However, we cannot rule out the possibility that these employees who remained working, despite their preexisting conditions, were also a selected, relatively healthy group, biasing results towards no association.
Effects of OPA on AMI, mediated by LTPA
Despite the fact that high OPA (RAS) negatively predicted high LTPA at baseline in our sample (ORadj 0.56, 95% CI 0.44–0.70 among men without IHD; ORadj 0.51, 95% CI 0.32–0.83 among men with IHD), our hypothesized mediating pathway from OPA to AMI via LTPA was not supported. This is probably due to the absence of an independent effect of LTPA on AMI after accounting for OPA and OPA-LTPA interaction.
In the current study, socioeconomic status as captured by education, cumulative measures for smoking and alcohol consumption, and psychosocial job factors are considered potential confounders and were adjusted for but biological factors were not. Different from PA measures that can be considered rather stable behaviors and are reflective of their activity levels for the past year or even a longer period of time before baseline interview, highly variable biological measures such as blood pressure and blood glucose assessed during baseline examination need to be considered mostly reflective of this moment in time and may have been influenced by past LTPA behavior. Therefore, biological factors were conceptualized as mediating variables on the pathways from OPA or LTPA to AMI and not adjusted for in the main analyses. Supplemental analysis with additional adjustment for these factors showed that the positive OPA–AMI association at low LTPA attenuated, suggesting indeed possible mediation by these factors. However, among men with IHD, the positive OPA–AMI associations persisted, despite the widened confidence intervals. Future studies can examine the possible mediating role of both LTPA and these biological factors (69, 70).
Strengths and limitations
The main strengths of our study include the prospective design, the representative sample of the population in Kuopio, long and complete register-based follow-up, and adequate covariate adjustment. Also, the use of a validated detailed occupational interview combined with objective measures of cardiorespiratory fitness in our main exposure variable (RAS) produced a better assessment of OPA compared to broad OPA categories or absolute EE obtained from most population-based surveys used in previous cohort studies. The assessment of LTPA accounted for the seasonal variability of LTPA among Finnish men by averaging LTPA over a 12-month period. Different analytic strategies were implemented to disentangle the impact of OPA and LTPA on AMI. The strategies included: accounting for cardiorespiratory fitness, using a priori cutoff-points based on recommended and established guidelines for OPA and LTPA, and conducting several sensitivity analyses including different modeling schemes and measures of PA domains. Finally, this is, to our best knowledge, the first study that used causal mediation analysis to examine the possible mediating pathway from OPA to AMI via LTPA.
Several limitations need to be addressed. Misclassification of OPA may be possible due to self-reporting rather than direct observations on type and duration of work activities and because EE assessment did not include upper extremity work or the handling of external loads. EE also did not account for the amount of static work and ambient temperature, leading to a possibly conservative measure of the actual amount of energy expended at work (14). Our relatively small sample size limited the statistical power to detect interaction between OPA and LTPA among men with IHD, possibly contributing to the relatively large P=0.24 for the multiplicative interaction term. Nonetheless, we could not rule out the possibility that our multiplicative OPA–LTPA interaction result was due to chance. Also, due to the lack of repeated cardiorespiratory fitness assessment at four years, we cannot compute a repeated RAS measure and further examine the change in RAS over time, leading to possible exposure misclassification. However, examination of repeated measures for absolute EE in this cohort revealed a relatively high correlation between baseline and four-year absolute EE (correlation coefficient: 0.77). Due to the lack of four-year RAS, our mediation analysis for estimating the path-specific effect of OPA on AMI via four-year LTPA could be subject to uncontrolled exposure (OPA) induced mediator-outcome (LTPA–AMI) confounding. Future work will involve conducting sensitivity analysis to check these results against the presence of such uncontrolled OPA-induced LTPA-to-AMI confounding that can introduce collider-stratification bias in the target OPA-to-AMI relation (25, 49).
Concluding remarks
Our study contributes to clarifying the unsettled complex roles of OPA, LTPA, and hence fitness in predicting AMI with and without preexisting conditions. We found that the impact of one PA domain on AMI depended on the level of the other PA domain on the multiplicative but not additive scale, when accounting for individual fitness. Our hypothesized mediating pathway from OPA to AMI via LTPA was not supported due to the absence of an independent effect of LTPA on AMI after accounting for OPA and OPA-LTPA interaction. Our results reaffirmed the need to develop PA recommendations that distinguish between OPA and LTPA (71), and take into account individual worker health status, aerobic fitness, and physical demands of the job when designing strategies for CVD prevention for working populations (15).