The World Health Organization (WHO) has defined contaminated sites as areas that have produced or might produce in the future an environmental contamination, which results or could result in human health impacts (1). The European Environmental Agency (EEA) estimates there are 342 000 contaminated sites (CS) in the 39 EEA countries on the basis of the soil contamination regulation; one third has already been identified and about 15% have been remediated (2). In Italy, a total of 57 sites have been defined as national priority contaminated sites (NPCS) in 2009, due to the relevance of soil and water contamination and the resulting potential health impact on the basis of European and national regulation. Epidemiological surveillance systems are essential in analyzing the health profile of populations residing in contaminated sites and for testing the associations between environmental exposure and health outcomes (3).
In the framework of the national strategic program “Environment and Health”, established by the Italian Ministry of Health and coordinated by the National Health Institute, the “SENTIERI project” (an epidemiological study of residents health in NPCS) was conducted with the aim of monitoring the health effects of environmental contaminations. The SENTIERI project measured an excess of deaths (9969 for all causes and 4309 for cancer) in the period 1995–2002 among residents in NPCS (4). In 18 NPCS served by a cancer registry, an excess of cancer incidence (about 9% among men and 7% among women) was observed in the framework of the same project (5). Among the contaminants listed by specific decrees that define the NPCS boundaries, asbestos (or asbestos-like fibres) was reported together with additional pollutant sources in 12 of the 44 sites investigated by the SENTIERI project, and as the only pollutant source in 4 (namely, the sites of Broni in Lombardy, Casale Monferrato in Piedmont, Bari in Apulia and Biancavilla in Sicily). In Italy, 3 748 550 tons of raw asbestos have been produced up to 1992 (year of definite asbestos ban), with a peak between 1976 and 1980 of >160 000 tons/year (6). For this reason, Italy is one of the countries more sensitive to the issues of monitoring and preventing asbestos-related health effects. Malignant mesothelioma (MM) is an uncommon neoplasm with high mortality that typically originates in mesothelial cells coating the serous cavities, mainly the pleura and the peritoneum and, to a lesser extent, also the pericardium and testicular tunica vaginalis. The risk of MM attributable to occupational asbestos exposure has been reported to range between 86–95%, according to epidemiological studies (7–9), but such percentages must be considered with caution as they are actually place- and time-specific.
The relevance of environmental exposure to asbestos for the occurrence of mesothelioma in Italy was recently estimated (10), and residences near asbestos-cement plants were frequently reported as a documented source of risk for mesothelioma in analytical epidemiological studies carried out in Casale Monferrato (11), Bari (12), Broni (13), La Spezia (included in the NPCS of Pitelli) (14). While the extent of occupational exposure to asbestos is expected to decrease in the next years, the contribution of different patterns of non-occupational exposures is likely underestimated due to exposure levels that are usually lower than in the workplace, although not negligible and reliably associated with the risk of mesothelioma (15).
As a consequence of the relevant and widespread use of asbestos in Italy, a permanent MM surveillance system has been active since 2002 through the National Register of Malignant Mesotheliomas (“Registro Nazionale dei Mesoteliomi – ReNaM” in Italian) at the National Institute for Insurance against Accidents at Work (INAIL). ReNaM performs active research of MM cases and asbestos-exposure assessment under standardized procedures and methods (16). Information about demographic, clinical, occupational, residential, and familial histories of MM cases are collected, and analytical studies about the incidence, epidemiological parameters of the diseases, territorial distribution of cases, and the modalities of asbestos exposure have been published (6, 10, 17). The aim of this study was to estimate the relative risk of MM incidence, provided by the ReNaM, and rank the risk of diseases for the population residing in NPCS.
Methods
The observed MM cases were extracted from the ReNaM archives in the period of diagnosis 2000–2011. In accordance with the law, the national register obtains data of incident MM cases from the Regional Operating Centres [Centri Operativi Regionali (COR) in Italian], currently established in all Italian regions. Diagnostic coding criteria are established by means of a 3-class scale of decreasing level of certainty: certain, probable, and possible MM (18). Occupational history, lifestyle habits, and residential histories are obtained using a standardized questionnaire completed by the subject or the next of kin. The classification of occupational exposure is qualitative, identified as: definite, probable, or possible. In the present analysis, the three levels of occupational exposure (definite, probable, possible) were considered together. Specific codes were assigned to environmental exposure (for a MM subject residing near a source of asbestos pollution, without any work-related exposure), familial exposure (when patients have lived with a cohabitant who has been occupationally exposed), and leisure activities exposure (other non-occupational exposures, likely due to leisure-time activities). Details are extensively described in the ReNaM national guidelines (19).
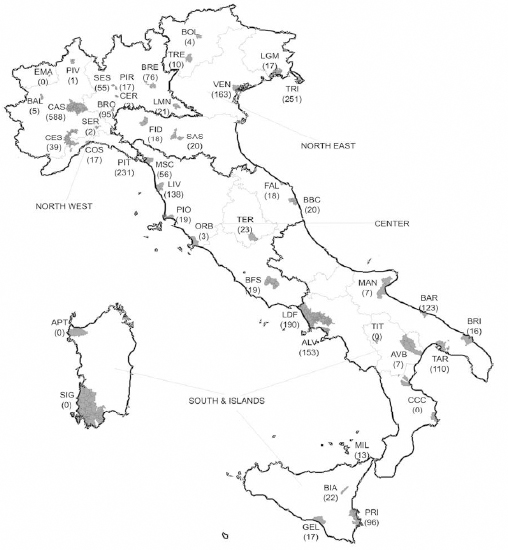
ReNaM is operational across all of Italy, but MM case lists from the regions of Sardinia and Calabria cannot be considered complete in the study period. For this reason, of the 44 Italian NPCS previously evaluated as eligible for the epidemiological assessment in the context of the SENTIERI projects, we excluded 3 sites (Sulcis-Iglesiente-Guspinese, Aree industriali Porto Torres in Sardinia, and Crotone in Calabria). Moreover, the corresponding COR did not detect any MM cases in 2 sites (Emarese in Valle d’Aosta and Tito in Basilicata), in the period 2000–2011, and they too have been excluded (figure 1). The time period of study has been restricted to 2001–2011 for the sites of Bacino idrografico fiume Sacco, Litorale Domizio Flegreo and Area Litorale Vesuviano, to 2005–2011 for the site of Bolzano, and to 2006–2011 for the site of Terni, due to unavailability of incidence data in the previous years.
Figure 1
Geographical distribution of the 39 national priority contaminated sites and number of malignant mesothelioma cases in the period 2000–2011.
Age-standardized incidence rates of mesothelioma (with direct method using the Italian population in 2010 as reference; all anatomical sites included) and trends over time for the overall NPCS were calculated in the period 2000–2011 by gender.
In each NPCS included in the analyses, the SIR for MM in any anatomical site (pleura, peritoneum, pericardium, and tunica vaginalis testis) were estimated for both genders separately. Gender- and age-specific rates of MM in the four Italian geographical macro areas (North-East, North-West, Centre, South-Islands) were calculated to derive expected numbers. The population of the macro areas was used as a reference in order to highlight the excesses of mesothelioma risk with a non-homogenous territorial distribution, taking into account the different industrial development across Italy. Therefore, each macro area must be considered individually, and the SIR emphasize the impact of asbestos exposure in every analyzed NPCS.
Confidence interval (CI) at 95% were estimated for each SIR according to Poisson distribution (20) and applying Byar’s approximation when >100 cases were observed (21).
Hierarchical Bayesian models, described elsewhere in detail (22), were specified on SIR. In brief, let Yi, the observed number of cases in the ith NPCS, follow a Poisson distribution with mean Eiθi, the expected number of cases times the parameter θi, the unknown relative risk (RR) for the ith NPCS. We assumed that for each area, log(θi) was independently drawn from a normal distribution with mean parameter following a weakly informative normal hyperprior distribution with zero mean and precision 0.0001 and precision parameter as inverse Gamma (23). Rank posterior distributions of RR were obtained from MCMC runs. The rank of the parameter of interest (RR θi) was computed at each iteration. The MCMC ran approximate the joint cumulative posterior distribution of RR F(θ|Y) and hence it was possible to approximate the posterior distribution [Ri|Y] ∀i and its summaries (for example the posterior mean as point estimate of the rank). Notice the posterior mean of the rank is usually not integer and is shrunken toward the mid-rank. From the posterior rank distribution, 80% CI can directly be obtained (24).
All statistical analyses were carried out by IBM SPSS statistics software (IBM Statistical Package for Social Sciences for Windows, Version 22.0. Armonk, New York, USA) and WinBugs14 (25).
Results
In the analysis of the 39 NPCS, 2683 MM cases (1998 men and 685 women) were detected in the period 2000–2011. The corresponding figure in Italy in the same period is 16 837 MM incident cases (4872 women and 11 965 men).
Pleural site is largely predominant (2535 cases, 94.5%), but peritoneal cases (142), pericardial (3), and tunica vaginalis testis (3) cases were also registered. The overall study findings are reported in table 1. A statistically significant (P<0.05) excess of mesothelioma incidence was estimated in 20 NPCS for men (of which 9 also for women), namely: Casale Monferrato (men and women), Cengio e Saliceto, Broni (men and women) and Pitelli in the North West of Italy; Venezia (men and women), Laguna di Grado e Marano and Trieste (men and women) in the North East; Massa Carrara, Livorno (men and women), Piombino, Falconara Marittima and Basso bacino Fiume Chienti in Central Italy; Litorale Domizio Flegreo, Area Litorale Vesuviano, Bari (men and women), Taranto (men and women), Milazzo, Gela, Biancavilla (men and women), and Priolo (men and women) in Southern Italy. A defect in male MM incidence cases was observed in the sites of Bolzano and Trento Nord (North East). Further excesses, but not statistically significant, were found in 8 NPCS for men, and 11 for women.
Table 1
Incident mesothelioma cases (N), standardized incidence ratio (SIR) and 95% confidence interval (95% CI) in Italian national priority contaminated sites (NPCSs) by gender.
| Region | NPCS | Acronym | Men | Women | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| N | SIR | 95% CI | N | SIR | 95% CI | |||
| North-Western Italy | ||||||||
| Piedmont | Balangero | BAL | 5 | 197.1 | 82.0–473.6 | · | · | ·· |
| Casale Monferrato | CAS | 340 | 910.7 | 816.5–1012.8 | 248 | 1338.1 | 1176.7–1515.4 | |
| Serravalle Scrivia | SER | 1 | 28.2 | 4.0–200.0 | 1 | 64.4 | 9.1–456.9 | |
| Pieve Vergonte | PIV | 1 | 43.6 | 6.1–309.2 | · | · | ·· | |
| Piedmont, Liguria | Cengio e Saliceto | CES | 31 | 165.8 | 116.6–235.7 | 8 | 98.0 | 49.0–195.9 |
| Lombardy | Cerro al Lambro | CER | 2 | 83.2 | 20.8–332.5 | 1 | 97.9 | 13.8–695.1 |
| Pioltello Rodano | PIR | 11 | 101.4 | 56.2–183.1 | 6 | 123.9 | 55.6–275.7 | |
| Sesto San Giovanni | SES | 37 | 80.6 | 58.4–111.2 | 18 | 81.5 | 51.3–129.3 | |
| Brescia Caffaro | BRE | 50 | 68.8 | 52.1–90.8 | 26 | 65.7 | 44.7–96.5 | |
| Broni | BRO | 52 | 1288.5 | 981.9–1691.0 | 43 | 2006.7 | 1488.3–2705.8 | |
| Laghi Di Mantova E Polo Chimico | LMN | 14 | 61.4 | 36.4–103.7 | 7 | 55.2 | 26.3–115.9 | |
| Liguria | Cogoleto Stoppani | COS | 14 | 154.6 | 91.6–261.0 | 3 | 69.9 | 22.5–216.7 |
| Pitelli | PIT | 201 | 445.4 | 385.9–511.4 | 30 | 124.5 | 87.1–178.1 | |
| North-Eastern Italy | ||||||||
| A.P. Bolzano | Bolzano | BOL | 4 | 17.5 | 6.6–46.7 | · | · | ·· |
| A.P. Trento | Trento Nord | TRE | 7 | 30.5 | 14.5–64.0 | 3 | 37.1 | 12.0–115.1 |
| Veneto | Venezia | VEN | 127 | 181.0 | 150.9–215.4 | 36 | 144.5 | 104.2–200.3 |
| Friuli Venezia Giulia | Laguna di Grado e Marano | LGM | 15 | 196.7 | 118.6–326.2 | 2 | 82.3 | 20.6–329.0 |
| Trieste | TRI | 209 | 374.5 | 325.4–428.9 | 42 | 204.4 | 151.0–276.5 | |
| Emilia Romagna | Fidenza | FID | 11 | 98.2 | 54.4–177.4 | 7 | 185.5 | 88.4–389.2 |
| Sassuolo - Scandiano | SAS | 18 | 80.6 | 50.8–127.9 | 2 | 29.0 | 7.3–116.1 | |
| Central Italy | ||||||||
| Tuscany | Massa Carrara | MSC | 46 | 243.4 | 182.3–324.9 | 10 | 149.6 | 80.5–278.1 |
| Livorno | LIV | 113 | 429.6 | 354.0–516.5 | 25 | 277.1 | 187.3–410.1 | |
| Piombino | PIO | 17 | 287.6 | 178.8–462.7 | 2 | 100.4 | 25.1–401.6 | |
| Orbetello | ORB | 2 | 88.0 | 22.0–351.8 | 1 | 128.7 | 18.1–913.7 | |
| Umbria | Terni – Papigno a | TER | 20 | 118.7 | 76.6–184.0 | 3 | 51.9 | 16.7–160.9 |
| Marche | Falconara Marittima | FAL | 17 | 401.0 | 249.3–645.1 | 1 | 72.2 | 10.2–512.6 |
| Basso Bacino Fiume Chienti | BBC | 17 | 128.2 | 138.1–357.4 | 3 | 70.9 | 38.7–372.5 | |
| Lazio | Bacino Idrografico Fiume Sacco b | BFS | 18 | 149.9 | 94.4–237.9 | 1 | 26.3 | 3.7–186.6 |
| Southern Italy and Isles | ||||||||
| Campania | Litorale Domizio Flegreo e Agro Aversano b | LDF | 154 | 150.0 | 130.4–180.0 | 36 | 105.5 | 79.5–152.7 |
| Area Litorale Vesuviano b | ALV | 133 | 336.3 | 281.5–398.5 | 20 | 152.7 | 98.5–236.6 | |
| Apulia | Manfredonia | MAN | 6 | 90.2 | 40.5–200.8 | 1 | 48.3 | 6.8–343.2 |
| Bari - Fibronit | BAR | 88 | 271.3 | 220.2–334.3 | 35 | 322.2 | 231.4–448.8 | |
| Taranto | TAR | 85 | 417.0 | 337.1–515.8 | 25 | 355.2 | 136.0–297.8 | |
| Brindisi | BRI | 13 | 154.9 | 89.9–266.7 | 3 | 107.0 | 34.5–331.7 | |
| Basilicata | Aree Industriali Val Basento | AVB | 7 | 173.4 | 82.7–363.8 | · | · | ·· |
| Sicily | Milazzo | MIL | 11 | 238.8 | 132.2–431.2 | 2 | 138.1 | 34.5–552.3 |
| Gela | GEL | 14 | 215.3 | 127.5–363.5 | 3 | 157.7 | 50.9–489.1 | |
| Biancavilla | BIA | 9 | 436.6 | 227.2–839.1 | 13 | 1941.4 | 1127.3–3343.5 | |
| Priolo | PRI | 78 | 447.7 | 358.6–559.0 | 18 | 330.2 | 208.1–524.1 | |
Trends in incidence for the period 2000–2011 reveal a peak in 2007 (age-standardized rate: 8.67 and 2.89 per 100 000 inhabitants among men and women, respectively), with a minimum in 2000 for men (5.79), whereas the trend is constant for women. In 2011, age-standardized rates are equal to 7.26 among men and 2.68 among women.
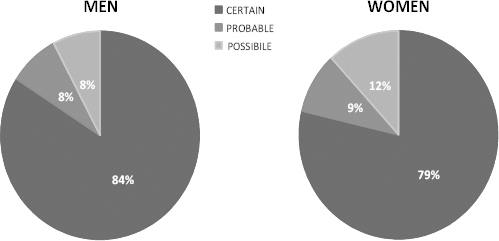
Most of the cases in the NPCS were diagnosed as certain MM (84% and 79% among men and women, respectively, figure 2).
Figure 2
Level of diagnostic certainty for malignant mesothelioma cases in Italian NPCSs in the period 2000-2011, by gender.
An individual questionnaire was used to investigate the modalities of asbestos exposure in 2150 MM cases (80.1% of the whole case list: 81.7% among men and decreasing to 75.6% among women). The amount of non-interviewed subjects vary according to the NPCS, with a decreasing trend from the North to the South of Italy, and non-negligible percentages (from 20% to 50%) in critical NPCS, such as Casale Monferrato, Litorale Domizio Flegreo or Area Litorale Vesuviano (table 2a).
Table 2a
Mesothelioma cases with exposure defined by interview in Italian national priority contaminated sites (NPCS), by gender. See table 1 for complete names.
Among cases evaluated for exposure, an occupational exposure to asbestos has been ascertained for 70.6% of cases from the overall case list, with a significant difference by gender (87% among men and 20% among women). The COR assigned non-occupational exposure (environmental, family and leisure-time related) to 409 cases (19%), with the most being represented by women (55%) (figure 3). In a percentage of MM, asbestos exposure could not be identified on the basis of available information, with different patterns between men (6%) and women (25%). The modalities of asbestos exposure by NPCS for all MM cases included in the analyses are reported in table 2b.
Figure 3
Modalities of asbestos exposure for malignant mesothelioma cases in Italian NPCSs in the period 2000–2011, by gender.
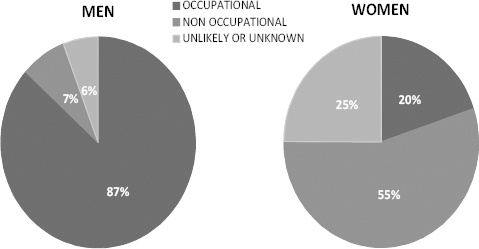
Table 2b
Modalities of asbestos exposure in mesothelioma cases with exposure defined by interview in Italian national priority contaminated sites (NPCS), by gender. See table 1 for complete names.
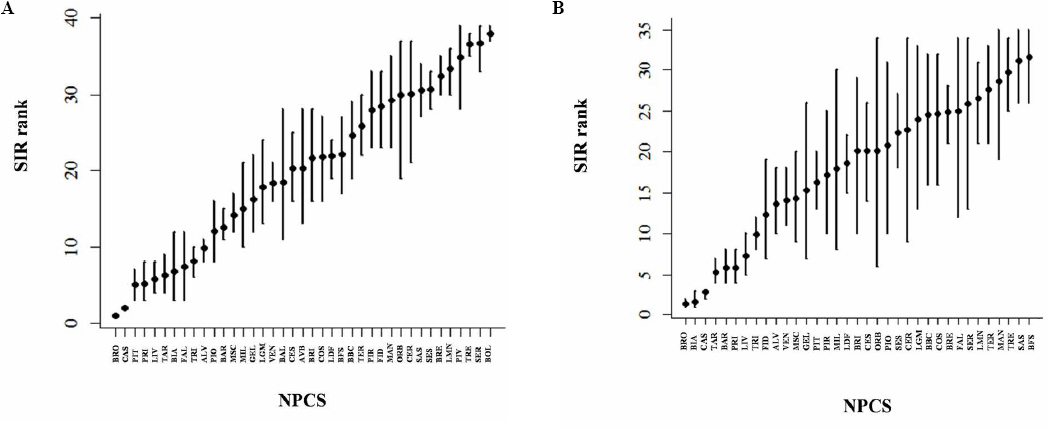
Profiling NPCS by Bayesian rank analysis highlights the firsts positions, consistently on both sexes (figures 4a, 4b). The site of Broni has the worse position for both genders. The sites of Casale Monferrato (both genders), and Biancavilla (only for women), resulted also among the higher positions, followed by the sites of Livorno (5th in men and 7th in women), Taranto (6th in men and 4th in women), Trieste (9th in men and 8th in women). Among the other sites, the degree of overlap of credible intervals is so large that it is impossible to clearly state an ordering by SIR.
Discussion
Through the SENTIERI approach, this study is the first attempt to combine the analyses of health profiles in the NPCS population with individual data from a cancer register (ReNaM), which includes the exposure and anamnestic history of the affected people.
The epidemiological surveillance of the health impact of contamination in NPCS contributes to the definition of priorities for remedial programs and public health interventions, and the SENTIERI project has been developed for this purpose. SENTIERI studied mortality, cancer incidence, and hospital discharge records of residents in NPCS specifically focusing on causes of death for which environmental exposure is suspected or ascertained to play an etiologic role (4). The epidemiological evidence of the causal association between diseases death and environmental exposure was classified a priori into three categories: sufficient (S), limited (L), and inadequate (I). The WHO defined this approach as a first-level stage in the description of the health status of residents in contaminated sites (1).
The systematic surveillance of mesothelioma incidence in Italy by means of a national register, which allows a qualitative assessment of asbestos exposure through the analysis of the entire occupational, residential and family history of each MM case, is an original and substantial experience in the international framework for managing and monitoring asbestos-related diseases (26). The occurrence of asbestos-related diseases in NPCS is relevant considering that in 10 out of 39 NPCS included in this analysis asbestos is one of the contaminants cited in the decrees defining the sites’ boundaries and the sources of pollution. In four sites (Casale Monferrato, Broni, Bari-Fibronit and Biancavilla), asbestos pollution is the only source of health risk identified in the decrees. In the Biancavilla site, the amphibole fluoro-edenite, mined from the local quarries and found in the soil and building materials, has been identified as responsible for the outbreak of mesothelioma observed in the town, as previously discussed (27).
Trends of incidence of mesothelioma in the overall NPCS show high figures among both genders that in 2011 are almost twice that of the national values (16). Such evidence is validated by the inclusion among the NPCS of sites such as Broni, Casale Monferrato, Biancavilla, whose incidence rates are very high due to the presence of important asbestos-cement plants in the first two sites and to the environmental exposure from the presence of a quarry with fluoro-edenite contamination in Biancavilla.
The reliability of diagnosis is similar in NPCS and in the rest of the ReNaM database, where the figures of the three levels of diagnostic certainty (“certain”, “probable”, “possible”) are: 82%, 9%, 9% for men, and 76%, 12%, 12% for women, respectively.
However, some critical limitations in the exposure assessment need to be discussed. The modalities of identifying asbestos exposure are not always fully uniform in the ReNaM network, and the percentage of interviewed subjects varies between 45% and 95% according to the COR, depending on their available resources and knowledge. As a consequence, the level of interviewed subjects in the NPCS is heterogeneous among different areas. It is one of the main limitation of this paper, and regards mainly NPCS such as Casale Monferrato, where the expositive history is certainly significant.
Also the procedures for coding and classifying the diagnosis of MM, established at national level, still lack of homogeneity among the CORs. Another limitation of this study is the use of the municipality of residence (at the time of diagnosis) to allocate the distribution of MM cases, and this is problematic when the subject resides outside the contaminated site where the past working activity was carried out.
Moreover, another limit is represented by the features of the ReNaM, which is designated by order of Italian law to record any mesothelioma case exclusively arising from the pleura, peritoneum, pericardium, and tunica vaginalis testis. No other anatomical sites are included. In perspective the ReNaM should extend the network of recording mesothelioma also to other asbestos-related neoplasms (eg, lung, larynx, ovary) (28).
A significant excess of MM cases has been found in 20 of the 39 NPCS selected for this study (in 9 sites for both men and women, in 11 sites only for men). Remarkably, in 12 of these (60%), asbestos was not included among the sources of pollution by the official decree. These sites were: Cengio e Saliceto, Venezia, Laguna Grado e Marano, Trieste, Falconara Marittima, Livorno, Piombino, Bacino Idrografico Fiume Sacco, Litorale Domizio Flegreo ed Agro Aversano, Taranto, Milazzo, and Gela.
The percentages of “definite”, “probable”, “possible” occupational exposure are 42.5%, 5.2%, and 8.9% respectively in the overall NPCS, corresponding to 38.4%, 5.7%, and 11% in the ReNaM database. The presence of occupational exposure to asbestos in a wide spectrum of economic activities, and not only in industrial settings where asbestos was directly used (asbestos cement plants, asbestos textile production, shipbuilding and repair, rail stock and rail carriage insulation, mining and work with friction products), has been repeatedly demonstrated in Italy (29) and elsewhere (30, 31). Our results support and confirm these findings, demonstrating that asbestos exposure is a relevant public health issue for a relevant number of contaminated sites, also without any mention of asbestos as direct cause of contamination.
In this study, we used 95% CI as opposed to the 90% CI generally applied in the SENTIERI project. That choice was made to show the range of uncertainty for risk estimators, reducing the use of CI as surrogate of hypothesis testing.
Reporting the NPCS ranking allowed a simple and immediate way to summarize the information about the profile of mesothelioma RR among the resident populations. Methodologically, it is important to underline that we provide an estimate of both the rank and its uncertainty. This is not trivial, and the degree of overlap in the CI is able to communicate to the reader the reliability of a given classification.
With regard to the methodology applied, the standardization procedure is motivated by the need to take into account not only the different age structure of the studied populations but also the presence of large-scale geographical variations. The ranking is therefore “relative” to the presence of large-scale geographical patterns. In Italy, the industrialization process included important difference between northern, central and southern regions, and asbestos exposures historically varied accordingly.
We therefore specified age and macro area-specific set of reference rates in order to gain sensitivity in the less industrialized macro areas. By observing the rank, the health impact of asbestos exposure in populations residing in the NPCS with a past history of direct asbestos use [asbestos cement plants (Broni, Casale Monferrato, Bari) or shipyards and harbours areas (Livorno, Taranto, Trieste, Piombino)] and the populations residing in territory with an environmentally diffuse contamination of fluoro-edenite (Biancavilla) emerged clearly (32).
In the present study about 20% of cases had a non-occupational exposure, predominantly among women (apart from Biancavilla, mainly in the sites of Casale Monferrato and Broni). The percentage of MM with a non-identified asbestos exposure is more relevant among women (25%) than men (6%). Such a difference underlines the need for tools investigating the modalities of exposure (ie, questionnaires) for the female population, which is more complex than the male one.
The ReNaM archive recently provided a reliable estimation of 10% of MM due to a non-occupational exposure to asbestos, based on more than 15 845 detected cases, of which 12 065 were individually interviewed (10). With respect to specific non-occupational exposures, the percentages of familial and environmental exposures are slightly higher than the corresponding figures in the ReNaM database for the same period (familial: 8% versus 5%; environmental: 10% versus 4% respectively). Non-occupational exposure to asbestos could be due to (i) naturally occurring contamination, (ii) the presence in residential areas of industrial sites previously involving asbestos use, (iii) the diffuse presence of asbestos industry by-products for insulation and for road and courtyard paving, (iv) cohabitation with exposed people, or (v) the accidental use of asbestos-containing materials. Such non occupational exposures have been analyzed extensively elsewhere (10). These various patterns pose different concerns with respect to the welfare protection framework and deserve special attention. Individuals were especially likely to be unaware of their exposure or of the associated hazard, as in the case of people living around industrial sources of asbestos pollution or with asbestos workers. The lower level of control over certain non-occupational circumstances, associated with the presence of asbestos in-situ, in buildings, make it possible for such exposures to persist. There is a need to discuss how to deal with compensation rights (currently reserved in many countries only for occupationally exposed people) for malignant mesothelioma cases induced by a non-occupational exposure to asbestos.
There is an open issue in Italy regarding MM cases of occupational origin that do not seek compensation (33). Furthermore, around 29% of MM cases due to non-occupational exposure in Italy has been found in NPCS and the percentage of non-occupational cases with respect to all cases investigated for exposure is higher in NPCS than in the ReNaM archive for the same period (19% versus 11% respectively). It is remarkable that recently the compensation of all MM cases, regardless of the modality or different patterns of exposures (naturally occurring contamination, industrial activities involving asbestos use existing in residential areas, cohabitation with exposed people, accidential use of asbestos-containing materials), has been implemented in France (34).
Our analyses confirm that asbestos pollution is a real concern in the NPCS, due not only to the occupational exposure of people working inside industrial plants where asbestos was directly used as a raw material or in workplaces where asbestos was present but also to the environmental exposure. Furthermore, non-occupational exposure to asbestos is largely the predominant cause of female mesothelioma cases in NPCS. A non-negligible proportion of mesothelioma cases identified at national level is concentrated in the NPCS: 16% of the whole ReNaM database (in the period 2000–2011), against an overall NPCS population representing the 9% of the Italian one. Moreover, the detection of significant mesothelioma excesses not only in NPCS, where asbestos is explicitly reported as a source of contamination, but also in a number of areas with other sources of pollution, confirms the wide range of activities and working and living environments affected by asbestos exposure, which are not restricted to the industrial sectors characterized by the direct use of this material.
The evaluation of occupational and environmental risk for people living in polluted areas, and the assessment of the risk produced by industrial settlements, could also be achievable by means of data available from surveillance systems of occupational tumors. The ReNaM-SENTIERI approach underlines the relevant role of such systems in the investigation of the associations between environmental exposures and health effects in polluted sites and reveals how this synergy can be successful for the permanent epidemiologic surveillance of occupational risks in contaminated sites.
In future, the aim is to extend the surveillance beyond mesothelioma to all occupational diseases in the NPCS by using the archives of compensated cases of occupational diseases available at INAIL. As a consequence, a permanent program for the epidemiological surveillance of occupational safety and health could be implemented in these areas where, when integrated with the analysis of environmental risks, results can improve and refine the interpretation of the health profile of populations affected by NPCS.