Hand eczema (HE) is a common disease with a negative impact on health-related quality of life (HR-QoL) (1, 2) and major socioeconomic implications (3). Onset of HE is influenced by individual susceptibility as well as environmental factors, in particular exposures at the workplace. In a Norwegian population study including respondents aged >20 years, the lifetime prevalence of HE related to work was recently reported to be as high as 4.8% (4). The median age for patients with recognized occupational HE is 37 years, and the prognosis of the disease often takes a chronic course, with the majority of patients with occupational HE reporting active disease after 12 years. Thus, due to the negative influence of the condition on individual well-being and work ability, and the high socioeconomic burden it places on society, focus should be placed on the prevention of occupational HE.
Skincare education has been introduced as secondary prevention for patients with occupational HE in Germany and comprises courses lasting up to several weeks (5–8). Patient training is, however, time-consuming and expensive, and evidence is needed to confirm efficacy. A total of three randomized controlled trials (RCT) have all indicated a positive effect of individual skincare education among HE patients (3–5). In these trials, the education was given individually, and the intensity of the intervention varied from a doctor-provided 30-minute educational briefing to a multidisciplinary intervention comprising several educational sessions involving different healthcare personnel. The degree of severity of HE differed between the trials. Moreover, one only comprised healthcare workers while the two others studies only included patients from a hospital setting.
The aim of the present RCT was to evaluate the effect of a skincare educational program versus treatment-as-usual among workers with notified occupational HE. The program comprised a one-time, 2-hour, group-based educational session, as well as written information with general and occupation-specific advice and a telephone hotline maintained by a nurse throughout the study period.
Methods
The methods are described in more detail in the appendix (www.sjweh.fi/show_abstract.php?abstract_id=3687).
PREVEX (PreVention of EXema) is an individually randomized, parallel-group superiority trial, investigating the pros and cons of a low-cost group counselling program versus treatment-as-usual among newly notified occupational HE patients. The three co-primary outcomes were sickness absence, HR-QoL, and severity. The trial (9) included patients with occupational HE notified to the Danish Labour Market Insurance in Region Zealand and the Capital Region of Denmark between 1 July 2012 to 30 November 2014. A questionnaire was sent by ordinary mail within 1–2 weeks of notification. Inclusion criteria were self-reported HE, written informed consent, and sufficiently completed information about profession and severity of HE in the questionnaire. Exclusion criteria were age <18 or >65 years, permanent exclusion from workforce, inability to understand Danish, and any serious medical condition that could interfere with the results (9).
Randomization and intervention
The Copenhagen Trial Unit performed the randomization. Participants were randomized individually 1:1 to the intervention versus the control group. Randomization was stratified according to age, HE severity, and profession (9).
The intervention comprised the following four elements: (i) A one-time, 2-hour, group-based education with alternating lecturing and workshops about skin-protective behavior, and a pamphlet with information from the course; (ii) job-specific counselling on work-related skin-protective behavior regarding allergens, irritants, and practical demonstrations of relevant gloves; (iii) information on rules and rights during an occupational injury; and (iv) a telephone hotline, to repeat information from the course, if required (9).
We had planned to offer eligible candidates (other than healthcare workers) a workplace visit, but since only 7.4% (14 out of 188) accepted the offer, it was withdrawn.
The control group received treatment-as-usual and had no access to the intervention.
Data collection and outcomes
The questionnaire at entry comprised questions on HE severity, HR-QoL, and self-reported occupation as well as questions on atopic disposition, knowledge of skin protection, risk behavior with respect to HE, treatment, and number of visits to the dermatologist. Severity was assessed by a validated photographic guide (10), and HR-QoL was assessed using the Dermatology Life Quality Index DLQI (11).
A follow-up questionnaire was sent out 12 months later, assessing the same variables. During the 12-month follow up, each participant was interviewed every 8th week (6 times total) regarding sickness absence. The primary outcomes were: (i) Self-reported number of days with sickness absence; (ii) HR-QoL assessed 12 months after inclusion (11); and (iii) self-evaluated HE severity assessed 12 months after inclusion (10). The explorative outcome was self-reported number of days with HE-related sickness absence during trial period.
Statistical analysis
Adjustment for multiple comparisons was done using Holm’s procedure (12). To ensure a power of ≥80% (risk of type 2 error 20%), a minimum of 742 participants were included (9). Intention-to-treat analysis was used.
Analyses
The primary as well as the exploratory results were obtained using adjustment by protocol-specified stratification variables (9). Unadjusted analyses were compared to the adjusted ones.Sickness absence was analyzed according to protocol using the Poisson distribution (9). Participants on parental leave were excluded (N=19).DLQI was analyzed using a negative binomial model fitted with the DLQI scores as a continuous outcome.Severity scores were analyzed, according to protocol, with a proportional odds model, cumulated over the lower ordered values.
Explorative outcome
Data regarding only HE-related sickness absence was analyzed using the same method as for the total amount of sickness absence.
Post-hoc analyses
As earlier trials (13, 14) showed a more beneficial effect of an educational intervention for patients with mild HE, we repeated the analysis of sickness absence data for participants with mild and severe HE, separately, at entry. This was done by adding an interaction term between the subgroup and intervention indicators.
Examination of baseline data indicated that healthcare workers differed from other occupations in that they reported less severe eczema and lower DLQI. Therefore, subgroup analyses were performed on healthcare workers and other occupations separately. This was done by adding an interaction term between the subgroup and the intervention indicators.
Statistical analysis was done with SAS (version 9.4, SAS Institute Inc, Cary, NC, USA).
The Danish Data Protection Agency approved the trial (journal number BBH-2011-33), which was registered at ClinicalTrials.gov Identifier: NCT01899287.
Results
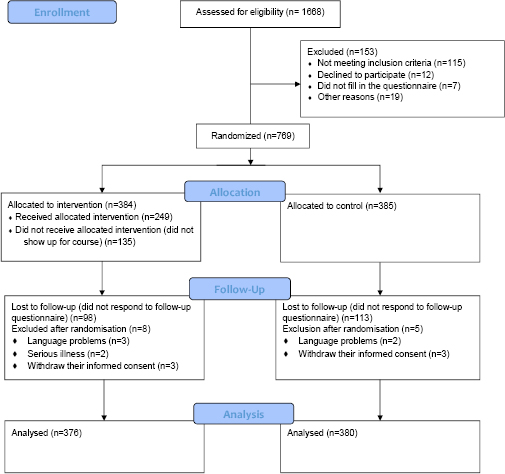
Of the 1668 individuals from the Labor Market Insurance who were invited to participate in the trial, 922 responded to the entry questionnaire (response proportion 55.3%). The non-responder group comprised more men (40.6% in non-responders and 29.4% in responders), while no marked differences were found regarding age or postal code. In total, 756 participants were included in the trial and eligible for the intention-to-treat analysis (Figure 1).
Entry characteristics of participants were evenly distributed in the intervention and control groups (table 1). The mean age in the intervention and control groups was 39.0 (SD 12.8) and 38.5 (SD 12.6) years, respectively. Of the participants who returned the follow-up questionnaire, 72.8% (398) had used topical corticosteroids (73.0% and 72.5% in the intervention and control groups, respectively), and 75.3% (412) had visits with a dermatologist (75.5% and 75.1% in the intervention and control groups, respectively).
Table 1
Characteristics of PREVEX trial participants in intervention and control groups at baseline. [SD=standard deviation; DLQI=Dermatology Life Quality Index; Q1=25th percentile; Q3=75th percentile].
|
Intervention (N=376) |
Control (N=380) |
|||
|---|---|---|---|---|
|
|
|
|||
| N | % | N | % | |
| Female | 274 | 72.9 | 260 | 68.4 |
| Severity (N=755 a) | ||||
| Clear (no symptoms) | 148 | 39.5 | 144 | 37.9 |
| Mild | 106 | 28.3 | 112 | 29.5 |
| Moderate | 85 | 22.7 | 81 | 21.3 |
| Severe | 27 | 7.2 | 34 | 8.9 |
| Very severe | 9 | 2.4 | 9 | 2.4 |
| DLQI (N=755 a) | ||||
| 0–2 | 105 | 28.0 | 121 | 31.8 |
| 3–5 | 104 | 27.7 | 91 | 23.9 |
| >6 | 166 | 44.3 | 168 | 44.2 |
| Atopic dermatitis (N=744) | 97 | 25.8 | 84 | 22.1 |
| Social class/status (N=749) | ||||
| Elementary school | 53 | 14.3 | 69 | 18.2 |
| Vocational education (etc) | 145 | 39.2 | 135 | 35.6 |
| Higher education (1–4.5 years) | 110 | 29.7 | 107 | 28.2 |
| Higher education (≥5 years) | 62 | 16.8 | 68 | 17.9 |
| Occupation (N=755) | ||||
| Healthcare personnel | 98 | 26.1 | 101 | 26.6 |
| Childcare | 45 | 12.0 | 36 | 9.5 |
| Kitchen workers | 30 | 8.0 | 43 | 11.3 |
| Cleaning personnel | 15 | 4.0 | 24 | 6.3 |
| Sales | 18 | 4.8 | 16 | 4.2 |
| Other (beauty, food industry, office, mechanics, etc) | 169 | 45.1 | 160 | 42.1 |
| Daily smoker (N=751) | 102 | 27.3 | 86 | 22.8 |
| Other diseases (N=674) | 114 | 35.9 | 124 | 35.7 |
Missing values
Of the 756 participants, 545 returned the follow-up questionnaire (response proportion 72.1%). When comparing participants lost to follow-up in regard to sex, age, severity, and postal code, no significant differences were found between the intervention and control groups. All missing values were evenly distributed between the two intervention groups (see Appendix, Table S1, www.sjweh.fi/show_abstract.php?abstract_id=3687).
Primary outcomes
Despite being 21% lower, there was no significant difference in total days of sickness absence in the intervention versus control group [95% confidence interval (CI) -55–40%, P=0.43] corresponding to estimate 0.79 (95% CI 0.45–1.40) (table 2).
Table 2
Crude and adjusted (adj) risk estimates for primary outcome: sickness absence and Health-Related Quality of Life (HR-QoL) Dermatology Life Quality Index (DLQI) score in the intervention compared with the control group (reference) as well as the intervention effect on sickness absence by healthcare workers and other occupations and significance test for difference of the intervention effect between healthcare workers and other occupations of the PREVEX trial. [HCI=confidence interval].
| Median | Percentiles |
Exponentiated Estimatecrude |
Exponentiated Estimateadj |
95% CIadj |
Interaction P-value a |
||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 25th | 75th | ||||||
| Rate of sickness absence | |||||||
| Intention-to-treat analysis | |||||||
| Intervention (N=349) | 0.008 | 0.00 | 0.03 | 0.80 | 0.79 b | 0.45–1.40 | |
| Control (N=361) | 0.011 | 0.00 | 0.04 | 1 | 1 | ||
| Interaction analysis | 0.07 | ||||||
| Healthcare occupation | |||||||
| Intervention (N=95) | 0 | 0 | 3 | 1.90 | 1.95 c | 0.60–6.37 | |
| Control (N=98) | 0 | 0 | 0 | 1 | 1 | ||
| Other occupations | |||||||
| Intervention (N=255) | 0 | 0 | o | 0.59 | 0.58 c | 0.30–1.15 | |
| Control (N=264) | 0 | 0 | 1 | 1 | 1 | ||
| HR-QoL (DLQI score) | |||||||
| Intention-to-treat analysis | |||||||
| Intervention (N=278) | 3.0 | 1 | 6 | 0.96 | 0.96 a | 0.82–1.13 | |
| Control (N=267) | 3.0 | 1 | 7 | 1 | 1 | ||
| Interaction analysis | 0.24 | ||||||
| Healthcare occupation | |||||||
| Intervention (N=76) | 2 | 1 | 5 | 1.10 | 1.15 c | 0.84–1.57 | |
| Control (N=78) | 2 | 0 | 4 | 1 | 1 | ||
| Other occupations | |||||||
| Intervention (N=201) | 4 | 2 | 7 | 0.92 | 0.92c | 0.76–1.11 | |
| Control (N=189) | 4.5 | 1 | 8.5 | 1 | 1 | ||
There was no significant difference in DLQI between the two groups (4% lower in the intervention group (95% CI -18–13%, P=0.67) corresponding to estimate 0.96 (95% CI 0.82–1.13) (table 2).
The ordinal odds of being in a worse severity category of self-reported HE were 15% lower in the intervention compared to the control group (95% CI -39–18%, P=0.34) corresponding to OR 0.85 (95% CI 0.61–1.18) (table 3).
Table 3
Crude and adjusted (adj) risk estimates for primary outcome: severity in the intervention compared with the control group (reference) as well as the intervention effect on severity, stratified by healthcare occupation and other occupations and significance test for difference of the intervention effect between healthcare workers and other occupations of the PREVEX trial. [OR=odds ratio; CI=confidence interval].
| Intervention | Control | ORcrude a | ORadj a | 95% CIadj | Interaction b P-value | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| N | % | N | % | |||||
| Intention-to-treat | ||||||||
| Clear | 99 | 37.2 | 94 | 37.0 | ||||
| Mild | 104 | 39.1 | 88 | 34.6 | 0.90 | 0.85c | 0.61–1.18 | |
| Moderate | 46 | 17.3 | 52 | 20.5 | 1 | 1 | ||
| Severe | 12 | 4.5 | 14 | 5.5 | ||||
| Very severe | 5 | 1.9 | 6 | 2.4 | ||||
| Interaction analysis | 0.044 | |||||||
| Healthcare occupation | ||||||||
| Clear | 29 | 38.2 | 39 | 52.7 | ||||
| Mild | 36 | 47.4 | 19 | 25.7 | 1.32 | 1.47d | 0.79–2.15 | |
| Moderate | 9 | 11.8 | 13 | 17.6 | 1 | 1 | ||
| Severe | 1 | 1.3 | 2 | 2.7 | ||||
| Very severe | 1 | 1.3 | 1 | 1.4 | ||||
| Other occupations | ||||||||
| Clear | 70 | 36.8 | 55 | 30.6 | ||||
| Mild | 68 | 35.8 | 69 | 38.3 | 0.80 | 0.69d | 0.47–1.02 | |
| Moderate | 37 | 19.5 | 39 | 21.7 | 1 | 1 | ||
| Severe | 11 | 5.8 | 12 | 6.7 | ||||
| Very severe | 4 | 2.1 | 5 | 2.8 | ||||
Explorative outcome
Despite being 20% lower in the intervention group [exponentiated estimate 0.80 (95% CI 0.32–1.99, P=0.34)], there was no significant difference regarding HE-related sickness absence.
In the repeated measures analysis, we found an increase in sickness absence over time in the control group [exponentiated estimate 1.09 (95% CI 1.01–1.18, P=0.03)]. In the intervention group, sickness absence increased less over time [estimate 1.02 (95% CI 0.93 - 1.12), P=0.72], but the difference between the two groups was not significant [exponentiated estimate 0.93 (95% CI 0.82–1.05)].
Post-hoc analyses
Per-protocol analysis
Analyses including only participants who had attended the course versus the control group were performed with respect to the three primary outcomes, which did not change the results (see Appendix, Tables S2 and S3, www.sjweh.fi/show_abstract.php?abstract_id=3687).
Mild compared to severe HE
A post-hoc analysis of sickness absence data comparing participants with mild HE [mild HE exponentiated estimate 0.73 (95% CI 0.35–1.53, P=0.40)] with participants with severe HE [severe HE exponentiated estimate 0.92 (95% CI 0.38–2.23, P=0.86)] was performed and showed no significant different effect of the intervention on the two severity groups (P=0.69).
Healthcare workers compared to all other occupations
When focusing on healthcare workers versus other occupations, there was an insignificant trend with respect to sickness absence that the effect of the intervention differed between the two groups. The healthcare workers experienced a detrimental effect versus a beneficial effect in other occupations (P=0.07). No significant difference regarding the effect of the intervention between healthcare workers and other occupations was found regarding DLQI scores (table 2). With respect to severity at follow-up, similar to sickness absence, the effect of the intervention differed between the two groups (detrimental effect on healthcare workers and beneficial effect on other occupations) (P=0.04), and a positive but statistically insignificant effect of the intervention was found for “other occupations” (P=0.06) (table 3).
Discussion
With respect to our three co-primary outcomes (sickness absence, HR-QoL, and severity of HE) we did not find effects of the intervention at follow-up. When comparing with previous randomized trials where the intervention was particularly effective among patients with mild HE (13, 14), we found it relevant to analyze data for patients with mild HE separately. However, this did not markedly influence the results.
Since sickness absence in PREVEX was self-reported, collected every second month, it was susceptible to recall- and social desirability-bias. In our trial, the mean value of sickness absence was 23.4 and 27.6 days in the intervention and control group, respectively, while the mean value of sickness absence in Denmark is 8.5 days (15). A Danish cohort study from 2004 found that 57% of occupational HE patients had sickness absence due to HE within the last year, and 19.9% had had >5 weeks (16), indicating that sickness absence may be a useful parameter for assessment of efficacy of an intervention. A previous intervention trial (17) evaluating effectiveness of integrated care likewise reported no effect on sickness absence but it included HE patients in general, and not only those with occupational disease, and an effect on sickness absence in these patients could be more difficult to detect.
A previous randomized clinical trial including healthcare workers with HE (13) found that education markedly and positively influenced the HR-QoL. In that trial, the intervention was a one-time, 30-minute individual patient education, and evaluation occurred after a period of five months, as compared to one year in our present trial. Two other trials with patients with more severe HE found no effect of the intervention with regard to DLQI (14, 17). Since planning of the present trial, a new and more sensitive method for assessment of HR-QoL among HE patients has been developed and should be the choice in future studies (18).
At follow-up, no significant difference was found with respect to severity, assessed by the photographic guide. This instrument is a validated tool known to correlate well with other objective scoring systems (19, 20). An advantage of this instrument is that it is patient-administered, favoring a higher response rate, as compared to an objectively assessed clinical score, which may however be more sensitive.
The relatively high number of participants with clear hands/no current hand eczema at entry may possibly have diluted the effect of the intervention with regard to severity.
The effect of an educational program as secondary intervention for patients with HE was previously studied in only three RCT (13, 14, 17). The interventions in these trials comprised, respectively: an individual one-time, 30-minute consultation with a doctor (13); one individual consultation with a dermatologist, four skincare education sessions with a nurse, and – if needed – consultation with a occupational physician (17); and a one-time individual nurse-led consultation and the possibility to contact investigators during the trial (14). In all trials, follow-up was after 5–6 months, and two of the trials reported a significantly positive effect on severity. Since the present trial found no effect on severity after one year, it is conceivable that the effect might attenuate. Although our intervention was not markedly less intensive than previous trials, it was group-based (with 1–14 participants), which may possibly explain the lack of imprinting a sufficiently lasting effect on the participants. A better strategy for a group intervention could be to either repeat the course with 6-months intervals or allocate participants according to occupation.
It may be speculated that the fact that only 66% of the participants in the intervention group attended the course could have diluted the effect of the intervention. However, per-protocol analysis including only attendees versus controls did not change the results. We assessed also HE-related sickness absence as an exploratory outcome, but no effect of the intervention was found.
Post-hoc subgroup analyses evaluating healthcare compared to non-healthcare workers indicated, interestingly, that the effect of the intervention tended to differ between different occupational groups. When focusing on healthcare workers, our results showed a markedly less positive influence of the intervention compared to other occupational groups with respect to sickness absence and severity at follow up. It is possible that healthcare workers already had a better knowledge of preventive and protective strategies and thus did not profit from the intervention. Rather the intervention increased awareness among the healthcare workers and resulted in higher sickness absence at follow-up.
Regarding our offer to conduct workplace visits, we assume fear over job security and additional work burden to be two of the primary reasons why 92.6% of the participants declined the opportunity.
The strengths of our trial were individual randomization and a successful randomization with two very homogenous groups regarding baseline values. Statistical analyses and drawing of conclusions were performed blinded to the intervention group. Trial design was chosen in order to minimize risk of systematic errors and risk of random errors (21, 22).
Drawbacks are the large proportion of missing data in primary outcomes and response rates of 55% and 72% for the baseline and follow-up questionnaires, respectively. It is unknown if inclusion of non-responding potentially eligible participants would have changed results. It was impossible to blind participants or educators regarding allocation to intervention or control group.
Concluding remarks
In the present trial, we found no effect of a simple, low-cost one-time, 2-hour, group-based skincare educational program versus treatment-as-usual on the primary outcomes (ie, sickness absence, HR-QoL and self-assessed severity of HE). Follow-up was after one year, and it is possible that a potential effect of the program had declined.
Explanations for this lack of effect could be that individual counselling may be more effective than group counselling, repeated programs may be needed, different occupations may need differently structured programs or, alternatively, the skin protection program simply does not work in this setting.
As post-hoc exploratory sub-group analysis indicated differing effects of the intervention between healthcare workers and other occupations, future research should assess the effectiveness of an educational intervention applied to specific occupations.