After almost 20 years of engineered nanomaterials (ENM) use in commerce, it is time to assess what is known about their health effects among workers. As the production of nanomaterials has grown, so has the workforce handling them. Within this growing workforce, the numbers of workers with adverse health effects from ENM exposure remains relatively unknown (1). The following factors can affect the assessment of ENM health effects: (i) the immense universe of potentially unique ENM and the great diversity in their toxic potential requires a unique assessment for each type, making a single overarching assessment of ENM health effects inappropriate; (ii) an assumed low extent of exposures due to a global attempt to promote responsible development of nanotechnology through preventive measures to control exposure to workers (2, 3); (iii) difficulty assembling study cohorts of similarly exposed workers due to decentralized manufacture and use of ENM (4, 5); and (iv) a lack of clarity on appropriate early indicators or biomarkers of adverse health effects.
A systematic review of the literature on the adverse effects of ENM in workers may be helpful in determining if effects exist and how deeply they have been investigated. Determination of the health effects and linkage to the exposure is a function of the extent to which workers have been exposed and the duration of their exposure. Since it is relatively early in the commercial history of ENM, the number of exposed workers, the extent of exposure, and the time since first exposure are generally small, leading to a low probability for adverse effects to have occurred, particularly chronic health effects. The ENM workforce is also widely dispersed and generally not very large in any involved workplace. Consequently, there have been few exposure assessment and epidemiological studies. Moreover, the heterogeneity of ENM in terms of physico-chemical variables is large. More precisely, the reactivity of tissues to ENM is highly dependent on particle morphology and surface features, related to their chemical growth histories (6).
The aim of this review was to identify health effects or early biological alterations that have occurred in the nanomaterial workforce. In the process, the review identifies the biomarkers related to such effects. Ultimately, the review seeks to identify knowledge gaps that require future investigation to help define strategies for suitable risk assessment and management processes.
Methods
The state of knowledge about the health impact of ENM in occupationally exposed populations was investigated through a systematic review of adverse effects reported in human studies and epidemiological investigations. Human case studies may provide preliminary information on possible adverse effects that have occurred in a single subject or a small group of subjects because of ordinary or accidental conditions of exposure. Epidemiologic investigations, on the other hand, are more useful and informative, as they can show possible relationships between ENM exposure and health effects.
Considering the multitude of ENM employed in workplace settings, and the early phase of knowledge concerning their possible adverse impact on human health, this review was primarily focused on the nine most widely used ENM identified in a recent WHO report and is based on the tonnes (t) of nanomaterials produced annually and used worldwide (7). These ENM include carbon black (9.6 million t); synthetic amorphous silica (1.5 million t); aluminum oxide (200 000 t); barium titanate (15 000 t); titanium dioxide (10 000 t), cerium dioxide (10 000 t); zinc oxide (8000 t); carbon nanotubes (CNT) and carbon nanofibers (CNF) (100–3000 t); and silver nanoparticles (NP) (20 t). “The term ENM refers to materials that have at least one dimension (height, width, length) that is smaller than 100 nanometers.” (7, p6)
The systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines for the period 2003–2018 (8). The year 2003 was selected as the starting date because it predated authoritative warnings of potential hazards of nanomaterials, such as those made by The Royal Society and Royal Academy of Engineering (9). The search used general databases at the University of Cincinnati (www.libraries.uc.edu), including the Web of Science, Scopus (Elsevier), PubMed, Academic Search Complete, Summon, and ProQuest. The following terms were employed to search for studies: “epidemiological studies”, “human studies”, “nanomaterial”, “nanoparticles”, “nanotubes”, “carbon black”, “carbon nanotubes”, “titanium dioxide”, “silver nanoparticles”, “cerium oxide”, “zinc oxide”, “aluminum oxide”, “synthetic amorphous silica”, and “barium titanate”.
A selection criterion for epidemiological studies included the use of a non-exposed or comparison group in the study. From previous investigations (10, 11), it was expected there would only be a small number of epidemiological biomarker studies. Most of the extant epidemiological studies of nanomaterial workers met the inclusion criteria and were included in the review, although some methodological criticisms emerged from previous analyses of such investigations (10–12). The aim of this review was to consider the findings in terms of commonality or divergence (10) between studies, pointing out coherent links with supporting literature, involving both human and animal subjects. Based on the findings of human and epidemiological studies, the scientific literature was further searched for information on mechanisms identified in or inferred from the results.
Results
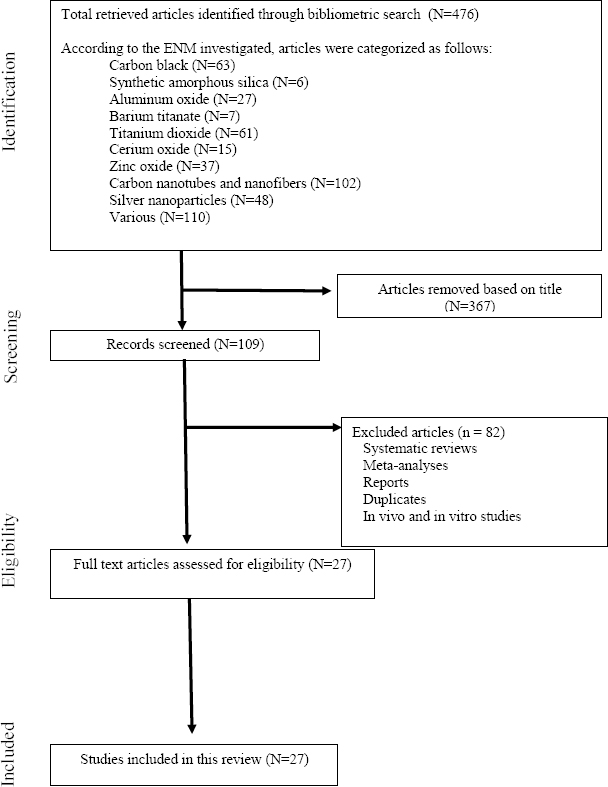
An electronic search of library databases yielded 109 unique references for human studies of which 27 were eligible to include in this review (figure 1). Of the 27 human studies, some studies involved more than one type of ENM and some used the same groups of workers. Table 1 describes principle findings from studies of nanomaterial workers, that is, workers exposed to contemporary ENM. The following sections will summarize results obtained in human case and epidemiologic studies by ENM category.
Table 1
Epidemiological and human case studies of nanomaterial workers. [NA=not available.]
| Nanomaterial a | Reference | Particle size | Workplace /operations | Sample size exposed/non-exposed workers | Findings |
|---|---|---|---|---|---|
| Carbon black | Zhang et al (20) | Primary size: 30-50 nm | Particle production | Exposed male workers (N=81); non-exposed male workers (N=104) | No changes found in chest x-rays images. |
| Significant, although no pathological, reduction of lung functional parameters: FEV1%, FEV1/FVC, MMF%, and PEF% in exposed workers compared to unexposed controls. | |||||
| IL-1β, IL-6, IL-8, MIP-1β, and TNF- alpha had 2.86-, 6.85-, 1.49-, 3.35-, and 4.87-folds increase in serum of carbon black workers compared to unexposed controls. | |||||
| Carbon black | Dai et al (21) | Primary size: 30-50 nm; agglomerates: 200-400 nm | Packing pure carbon black | Exposed male workers (N=106); non-exposed controls (N=112) | Peripheral blood eosinophil count in exposed workers increased by 30.8% compared to unexposed controls. |
| Silica nanoparticles b | Song et al (34) | 30 nm | Spray painting | Female workers in spray painting for 5-13 months (N=8) | Seven female workers claimed shortness of breath, pleural and pericardial effusion, pulmonary inflammation (2 died). |
| Silica nanoparticles b | Song et al (35) | Primary size: 20-21 nm | Spray painting | Exposed workers employed in spray painting for 5-13 months (N=8) | In the early stages (within 3 months of disease onset), ENM 20–21 nm in diameter were observed and found in the cytoplasm, nuclei and organelles of macrophages, pulmonary microvessels, pulmonary vascular endothelial cells, and microlymphatic vessels. |
| In the late disease stage (18 months, just prior to death), few ENM were observed in pulmonary cells and interstitial tissue, as well a few in macrophages. | |||||
| Titanium dioxide | Ichihara et al (68) | 46-562 nm <300 nm | Process and handle | Exposed workers (N=16); no controls | Handling <300 nm sized TiO2-ENM might affect heart rate variability in workers. |
| Titanium dioxide | Pelclova et al (69) | 80% of airborne particles were <100 nm c | Production and research | Exposed production workers (N=32); exposed research workers (N=4); non-exposed controls (N=45) | The concentration of titanium in EBC may serve as a direct exposure marker in workers producing TiO2. |
| The markers of oxidative damage of nucleic acids and proteins in the EBC were significantly higher in the more exposed production workers, than in the research workers and unexposed controls. | |||||
| Titanium dioxide | Pelclova et al (67) | 80% of particles <100 nm c | Manu-facturing | Workers exposed to TiO2 (N=30); office employees (N=22); non-exposed controls (N=45) | Leukotrienes (LT) B4, C4, E4, and D4 were all elevated in the EBC samples of exposed workers compared to controls and were directly correlated with Ti concentrations. |
| Office workers had higher LTB4 in EBC and higher levels of the fractional exhaled nitric oxide. | |||||
| Titanium dioxide | Pelclova et al (70) | 80% of particles <100 nm c | Manu- facturing | Exposed workers (N=34); non-exposed controls (N=45) | All 11 markers of lipid oxidation analyzed were elevated in production workers relative to the controls. A dose-dependent association was detected between exposure to TiO2 and markers of lipid oxidation in the EBC. |
| No elevation was evident in the urine samples. | |||||
| Titanium dioxide | Pelclova et al (71) | 80% of particles <100 nm c | Manu- facturing | Exposed workers (N=22); non-exposed controls (N=14) | Nine markers of lipid oxidation were elevated in the EBC of office employees relative to controls; only 8 isoprostane and aldehyde C12 were not increased. |
| Urine concentrations of the oxidative stress markers were not elevated relative to the control group. | |||||
| Titanium dioxide | Pelclova et al (64) | 70-82% of airborne particles were <100 nm | Production and research | Production workers- high risk of exposure (N=16); research | Particles of rutile and/or anatase were found in the EBC of exposed workers in 8/20 (40%) of the pre-shift and 14/20 (70%) of the post-shift samples. |
| Workers- medium risk of exposure (N=4); non-exposed controls (N=20) | The mean concentration of titanium in the EBC samples in production workers was 24.1 ± 1.8 μg in the pre-shift and 24.1±1.9 μg in the post-shift, while in controls mean concentration was ≤ the limit of determination. | ||||
| Titanium dioxide | Zhao et al (65) | 39% of particles <100 nm | Exposed workers (N=83); non-exposed controls (N=85) | Lung damage biomarkers: serum surfactant protein-D was significantly lower in exposed workers compared to unexposed controls. | |
| Cardiovascular disease biomarkers: vascular cell adhesion protein 1, (VCAM-1), intercellular adhe sion molecule 1 (ICAM-1), and low density lipoprotein were higher, while total cholesterol was reduced in exposed workers compared to unexposed controls. | |||||
| Inflammation and oxidative stress markers: superoxide dismutase (decreased) and malondialdehyde (increased); inflammation markers: interleukin (IL)-8, IL-6, IL-1β, TNF-α, and IL-10 were increased and significantly associated with occupational exposure to nano-TiO2. | |||||
| Multi-walled carbon nanotubes | Fatkhutdinova et al (123) | External diameter: 8-15 nm; internal diameter 4-8 nm; length: ≥2 µm | Nano-material manu- facturing | Exposed workers (N=10); non-exposed controls (N=12) | Inflammatory biomarkers (serum): IL-1β, IL-4, and TNF-α were significantly elevated in the MWCNT exposed group. |
| Inflammatory biomarkers (sputum): profibrotic inflammatory biomarkers, such as cytokines IL-1β, IL-4, IL-5, IL-6, IL-8, TNF-β and KL-6 were significantly higher in exposed workers compared to unexposed controls. | |||||
| Multi-walled carbon nanotubes | Lee et al (121) | NA | Nano-material manu- facturing | Exposed workers (N=9); non-exposed office employees (N=4) | Oxidative stress biomarkers: increased levels of malondialdehyde (MDA), 4-hydroxy-2-hexonal, and N hexanal in the exhaled breath condensate of exposed workers compared to unexposed controls. |
| Multi-walled carbon nanotubes | Shvedova et al (127) | 2-10 nm × micrometer | Manu- facturing | 8 exposed workers; 7 non-exposed controls | Alterations in non-codingRNA and mRNA expression profiles showed a set of miRNAs and their target genes with functions in cell cycle regulation/progression/control, apoptosis and proliferation. |
| The identified pathways and signaling networks also revealed MWCNT potential to trigger pulmonary and cardiovascular effects as well as carcinogenic outcomes. | |||||
| Multi-walled carbon nanotubes | Vlaanderen et al (122) | NA | Manu- facturing | Exposed workers (N=22); non-exposed controls (N=39) | Respiratory effect biomarkers: decreased Fractional Ehaled NO concentrations. |
| Immunological biomarkers: increased concentration of C-C motif ligand and 20, basic fibroblast growth factor (FGF), and soluble IL-1 receptors in EBC. | |||||
| Hematological alterations: significant depression in neutrophils and significant elevation in monocytes, mean platelet volume, immature platelet fraction, and immature reticulocytes fraction with increasing exposure to MWCNT. | |||||
| Multi-walled carbon nanotubes | Ghosh et al (128) | 200 nm -100 µm | Manu- facturing | Exposed workers (N=24); Non-exposed controls (N=43). Follow-up (after 4 months): exposed workers (N=11); Non-exposed controls (N=4). | Significant changes in methylation of at least one or more CpG sites were observed for all the genes studied (ENMAT/ATM, SKI, DNMT1, and HDAC4) in MWCNT exposed workers. |
| Multi-walled carbon nanotubes | Kuijpers et al (125) | NA | Manu- facturing | Exposed workers (N=22); Unexposed controls (N=42). | Dose-dependent upward trend in the concentration of endothelial damage marker ICAM-1 in MWCNT exposed workers compared to unexposed controls. |
| Follow-up (after 5 months): exposed workers (N=13); Non-exposed workers (N=6) | |||||
| Carbon nanotubes / carbon nanofibers | Beard et al (124) | 1.5-110 nm × 3.3 µm -1 mn | Manu- facturing /handling | Exposed workers (N=90) | Biomarkers associated with at least three CNT/carbon nanofiber metrics in sputum: type IV collagenase/matrix metalloproteinase-2 (MMP-2), IL-18, glutathione peroxidase (GPx), myeloperoxidase, and superoxide dismutase (SOD). |
| In blood: MMP-2, matrix metalloproteinase-9, metalloproteinase inhibitor 1/tissue inhibitor of metalloproteinases 1, 8-hydroxy-2′-deoxyguanosine, GPx, SOD, endothelin-1, fibrinogen, ICAM-1, VCAM-1, and von Willebrand factor. | |||||
| Carbon nanotubes / carbon nanofibers | Dahm et al (2) | 1.5-110 nm 3.3µm -1nm | Manu- facturing /handling | Exposed workers (N=90) | Only 18% of investigated workers had CNT/F present in sputum samples. |
| When subdivided in industry, 18%, 11% and 27% of the participants within the primary manufacturing, hybrid, and secondary manufacturing facilities had CNT/F present in their sputum. | |||||
| Carbon nanotubes / carbon nanofibers | Schubauer-Berigan et al (117) | 1.5-110 nm 3.3µm -1nm | Manu- facturing /handling | Exposed workers (N=108) | Inhalable elemental carbon (EC) concentration and duration of work with CNT/F were positively associated with the development of respiratory allergies. |
| Resting heart rate (RHR) was positively related to inhalable and respirable EC concentrations, while hematocrit counts showed a positive relationship with CNT/F structure count concentrations. | |||||
| Silver | Lee et al (147, 148) | <100 nm 20-30 nm | Manu-facturing | Exposed workers (N=2) | Biological monitoring of silver: 0.034 and 0.0135 µg/ml in blood; none in urine. |
| Various nanomaterials (Ag, iron-oxide, nanogold, CNT, TiO2, SiO2) | Liao et al (36) | <100 nm | Manu- facturing 4-11 years | Exposed workers (N=124); non-exposed controls (N=77) | The decreases in the changes in SOD, GPx between baseline and the 6-month follow-up in the exposed group were significantly greater than in unexposed controls. |
| VCAM between baseline and the 6-month follow-up in the exposed group was significantly greater than in the unexposed controls. | |||||
| Various nanomaterials (CNT, TiO2, SiO2, and others ie, nanoresins, Ag, nanogold, nanoclay, nanoalumina, and metal oxides) | Liou et al (37) | <100 nm | Manu-facturing handling | Exposed workers (N=227); non-exposed controls (N=137) | Increased levels of anti-oxidant enzymes (SOD, GPx) were found in exposed workers (directly and indirectly handling nanomaterials) compared to unexposed controls. |
| Increased expression of cardiovascular markers: fibrinogen, IL-6, ICAM were found in exposed workers compared to unexposed controls. | |||||
| Titanium dioxide, silica dioxide, indium tin oxide (ITO) e | Liou et al (38) | <100 nm | Manu-facturing | Workers exposed to TiO2- (N=26), SiO2- (N=31) | Exposure to TiO2-, SiO2-, and ITO-ENM resulted in significantly higher oxidative biomarkers such as urinary 8-OHdG and EBC 8-isoprostane. |
| ITO-ENM (N=30); non-exposed controls (N=43) | Global DNA methylation levels were significantly lower in SiO2 and ITO exposed group than in unexposed controls. | ||||
| Titanium dioxide, silica dioxide, indium tin oxide | Liou et al (39) | <100 nm | Workers exposed to TiO2 (N=32); SiO2 (N=39); ITO- ENM (N=56); non-exposed controls (N=100) | Exposure to TiO2, SiO2, and ITO resulted in significant lower antioxidant enzymes (GPx and SOD) and higher oxidative biomarkers 8-hydroxydeoxyguanosine (8-oxodG) in urine, blood and white blood cells than in unexposed controls. | |
| Various (CNT, titanium dioxide, silver, indium tin oxide) | Wu et al (66) | NA | 13 factories manuf- acturing ENM | Exposed workers (N=241); non-exposed controls (N=196) | Nano TiO2 exposure was significantly associated with fractional exhaled NO as a diagnostic test of airway inflammation. |
Carbon black
Carbon black is a generic term for a high volume commercial material of many different types of amorphous carbon with a wide range of particle sizes. Carbon black has been in commerce for over a century (13). Particle size may vary with use. Primary particles range from 1– 500 nm; however, particles generally aggregate to sizes of 50–600 nm and, in turn, aggregates agglomerate to micron sizes (14). Carbon black particles of nanoscale size are manufactured by various vapor-phase processes, pyrolysis, and partial combustion or thermal decomposition of gaseous or liquid hydrocarbons (14). Non-malignant respiratory morbidity in terms of pulmonary function decrement and respiratory symptoms was seen in carbon black workers and was strongly correlated with the levels of exposure (15–19). The International Agency for Research on Cancer (IARC) deemed as inconsistent epidemiologic data of carbon black workers, which show some indications of lung cancer (14). The IARC did find a more consistent pattern of lung cancer in animal studies supported by mechanistic investigations (14) and concluded there was sufficient evidence in experimental animals for carcinogenicity. Consequently, carbon black was classified as a possible carcinogen to humans (14). However, the studies generally did not identify particle size. It cannot be excluded that some of the particles could be in the nanoscale range.
Although major concerns exist regarding the potential impact of human exposures to nano-sized carbon black particles, limited human data are currently available. In relation to clinical parameter alterations, in a cross sectional epidemiological study on carbon black workers, a significant reduction of lung functional parameters was evident compared to unexposed controls (20). This reduction of lung functional parameters included the percent predicted forced expiratory volume in 1 second (FEV1%), FEV1/ forced vital capacity (FVC), percent predicted maximal mid-expiratory flow curve (MMF%), and percent predicted peak expiratory flow (PEF%). None of the exposed workers had pathologically low values of these parameters (20). The mean concentration of carbon black in this study, measured by personal samples was 14.90 mg/m3, which is 4.26 fold higher than the current TLV of 3.5 mg/m3 (20).
In a subsequent investigation, the same group of researchers analyzed possible changes exerted by carbon black ENM-occupational exposure on hematological indices (21). Their results showed a significant increase in peripheral blood eosinophil count in exposed workers as a sign of nanomaterial induced inflammation (20). These findings were supported in the previous study of other possible pro-inflammatory biomarkers, which found a significant increase in circulating pro-inflammatory cytokines, ie, interleukin (IL)-1β, IL-6, IL-8, macrophage inflammatory protein (MIP)-1β, and tumor necrosis factor (TNF)-α in exposed carbon black workers compared to controls (20). These results are in line with those reported in animal investigations demonstrating pulmonary inflammation (19, 22–24) associated with a severe tissue damage (25, 26) and a significant increase in serum pro-inflammatory biomarkers, including pro-inflammatory cytokines in treated animals (20, 27–29).
Although further investigation seems necessary, such preliminary results can suggest that carbon black nanomaterials may be responsible, at least in part, for the reduction of pulmonary function and inflammatory response detected in exposed workers. Concerning a possible dose–response relationship, the limited available data on environmental exposure concentrations, as well as the lack of biological monitoring information on occupational exposure, prevent the achievement of definite conclusions in that regard. Pulmonary functional alterations observed in workers exposed at concentrations greater than the adopted TLV underlines the importance of investigating high levels of exposure. However, from a precautionary perspective, preliminary knowledge regarding the toxicological behavior of ENM also requires investigation of possible adverse effects on workers exposed to low concentrations for long periods.
Synthetic amorphous silica (SAS)
Synthetic amorphous silica has been in commerce for >80 years. This silica polymorph is an intentionally manufactured material that does not contain measurable levels of crystalline silica (30, 31). It is a nanostructured material consisting of aggregates and agglomerates of primary particles that are generally <100 nm (32). There are three categories of SAS based on manufacturing: pyrogenic (7–50 nm); precipitated (5–100 nm), and gels (3–20 nm) (30). A review of epidemiological studies and case reports of workers exposed to synthetic amorphous silica going back to 1932 failed to show conclusive evidence of fibrosis, but the data were limited (30, 33). The studies did not exclude the risk of chronic obstructive pulmonary disease (COPD) and emphysema, and none are informative with regard to carcinogenicity (30, 33).
For production workers exposed to nanoscale SAS, no explicit adverse effects have been found in recent epidemiological cross-sectional studies. However, one case series, Song et al (34), described seven female workers, employed in the same department of a print plant and exposed to ~30-nm-sized silica ENM in a polyacrylic ester. The workers had no protective measures for periods as long as 5–13 months. The workers developed shortness of breath and pleural effusions. Pathological examinations of the patients’ lung tissue displayed nonspecific pulmonary inflammation, pulmonary fibrosis and foreign-body granulomas of the pleura (34). Electron microscopy and energy dispersive x-ray analysis identified and characterized silica-ENM primarily in macrophages, pulmonary micro-vessels, and pleural effusions. The findings suggest that these ENM may have contributed in part to the illness reported by these workers, although it is not clear if these ENM were amorphous silica (35). A cross-sectional study of SiO2-NP exposed workers had significantly greater cardiovascular related biomarkers, heart rate variability, vascular cell adhesion molecule (VCAM), and intercellular adhesion molecule (ICAM) than controls (36).
One hypothesis-generating study (37) demonstrated that workers employed in 14 factories in Taiwan who handled multiple ENM, including nanoscale silica, had reduced serum levels of antioxidant enzymes. Considering the key role of ENM induced reactive oxygen species (ROS) in tissue toxicity, possible biomarkers of early oxidative stress were specifically investigated in a subset of workers (N=37) using SiO2-ENM (12–200 nm) (38). In this context, measurements of 8-hydroxydeoxyguanosine (8-OHdG) in urine (38, 39), plasma, and white blood cells (39) and exhaled breath condensate levels of 8-isoprostane (38) were significantly higher compared to the controls, while levels of anti-oxidant enzyme activities were significantly decreased in the exposed group compared to controls (38). When two examinations were performed on workers handling SiO2-ENM at baseline and 6-month follow up, the depression of serum antioxidant enzymes levels, ie, superoxide dismutase and glutathione peroxidase, were significantly greater in exposed groups compared to controls (36). As regards epigenetic alterations, which may affect genetic regulation and cellular differentiation, a significantly lower global level of DNA methylation was observed in peripheral white blood cells of workers handling SiO2-ENM compared to controls. Interestingly, such alterations were inversely correlated with the urinary and white blood cell 8-OHdG concentrations (38).
These epidemiological findings (36–39) have been confirmed in animal models in which exposure to nanoscale SAS particles was reported to induce low systemic and negligible pulmonary toxicity (40–42). The particle’s ability to distribute in lungs, lymphatic tissues, and major organs of excretion, such as the liver and kidneys, following inhalation exposure was limited (43, 44). However, there is evidence from in vivo studies that one type of SAS, fumed silica, has been shown to generate cytotoxicity and pro-inflammatory effects (31, 45–47).
Overall, epidemiological findings may support that one of the principal mechanisms of SiO2-ENM toxicity is the generation of reactive oxygen species and oxidative injury. This is the major mechanism by which ENM may induce adverse health effects and, in such effects, possible biomarkers may be found. Innovative effect biomarkers may be identified in investigations focused on ENM-induced epigenetic effects. These may include not only methylation changes at the global and repeated DNA level but also possible alterations in response to ENM exposure at specific loci. Additionally, these investigations indicate that oxidative DNA damage may have a role in inducing such phenomena. However, these latter issues need to be clarified with further research. Finally, no data are currently available concerning the exposure levels potentially responsible for such described alterations. The pathological alterations that occurred in workers exposed to ENM who were not wearing personal protective equipment (34, 35) suggest the need to verify the role of collective and individual exposure controls in managing the risk of possible ENM-induced adverse health effects.
Aluminum oxide
Aluminum oxide results from refined bauxite, which is subsequently reduced to aluminum (48). There have been no epidemiological studies of intentionally manufactured aluminum oxide nanomaterials. However, there have been occupational studies that have shown that inhaled aluminum oxide particles (size unspecified) are linked to pulmonary fibrosis, asthma, chronic obstructive lung diseases and possibly lung cancer (49–52). Ultrafine particles have been identified in primary aluminum smelters and pot rooms (53). Concerning animal data, pulmonary inflammation and cytotoxicity (54), an increase in the number of immune cells in BAL fluid (55, 56) and levels of IL-6, macrophage inflammatory protein-1α (MIP-1α), and monocyte chemoattractant protein-1α (GM-CSF) were reported after aluminum oxide ENM inhalation. Overall, the lack of epidemiological studies on possible adverse health effects prevents definite conclusions on the impact that aluminum oxide ENM may have on the health of exposed workers and possible markers indicative for early detection of biological alterations.
Barium titanate
Barium titanate (barium titanium trioxide) is a member of the large family of compounds with the general formula ABO3, known as perovskites (57). Barium titanate is a widely used electro-ceramic material that is increasingly used in biology and medicine. It is produced in a variety of ways and with a broad range of particle sizes, including some <100 nm (57, 58). There appears to be neither documentation of occupational exposures to barium titanate nanomaterials nor animal inhalation studies. However, a study of mice injected with hydroxyapatite-barium titanate composites (nano to submicron agglomerates) for implant testing indicated an absence of any inflammatory or adverse reactions (59). Considering the widespread application of this compound at the nanoscale, it seems important to plan future studies investigating early biological alterations in occupationally exposed populations.
Titanium dioxide
There are four naturally occurring titanium dioxide polymorphs: rutile, anatase, brookite, and titanium dioxide (B) (14). Particle size plays an important role in many TiO2 applications. The major use of TiO2 is in pigments where particles in the size range of 200–300 nm are generally employed. Many other uses, eg, in the electronic field, involve particles <100 nm (14). Epidemiological studies of TiO2 production workers show limited evidence of malignant or nonmalignant health effects, although the particle size was not specified (14, 60, 61). In 2010, the IARC reviewed epidemiologic data and found inadequate evidence to classify TiO2 as a human carcinogen (12). However, lung tumors observed in rats following chronic inhalation of nano-sized TiO2 included squamous cell keratinizing cysts, bronchoalveolar adenocarcinomas, and squamous cell carcinomas (62, 63). These findings led the IARC to consider animal data sufficient for an evaluation of “possibly carcinogenic to humans” (14).
Epidemiological studies performed on nano-TiO2 production workers are generally cross-sectional in nature. These studies have not shown a clear pattern of health effects, although some biological alterations emerged as possible indicators of exposure and early effect. Pelclova et al (64) demonstrated that particles of rutile and/or anatase could be detected in the exhaled breath condensate (EBC) of exposed workers. The content of the metal in their breath was significantly higher compared to controls, suggesting a method of measurement to assess exposure to TiO2-ENM.
Zhao et al (65) recently investigated the pulmonary effects induced by TiO2-ENM in workers exposed in a packaging workshop to identify possible functional alterations and biomarkers associated with exposure (estimated mass concentration of 1.22 mg/m3). The authors found that the observed and predicted values of FVC, FEV1, peak expiratory flow (PEF), and forced expiratory flow (FEF) 25–75%, were significantly reduced in exposed workers compared to the controls. In this study, pulmonary function test alterations were also confirmed by the decrease in serum levels of surfactant protein-D levels, which may be a preclinical lung damage biomarker caused by cell injury and/or decrease in number of type II alveolar epithelial cells. As a possible diagnostic test of airway inflammation, the fractional exhaled nitric oxide (FENO) measurement was significantly increased in workers exposed to TiO2-ENM (66). Additionally, leukotriene levels were significantly elevated in the EBC of exposed workers relative to controls and well correlated with workplace Ti concentrations (67). The possibility of using such biological alterations to monitor the early effects of nano-TiO2 exposures on workers deserves further investigation.
Regarding the cardiovascular effects, Ichihara et al (68) demonstrated that exposure to TiO2-ENM was associated with heart rate variabilities in workers involved in processing and handling such nanomaterials. Moreover, to support a possible role of TiO2-ENM in inducing cardiovascular alterations, Liao et al (36) and Zhao et al (65) demonstrated increased levels of VCAM-1, ICAM-1, and low-density lioprotein (LDL) as possible cardiovascular early disease biomarkers.
Concerning the role that systemic inflammatory as well as oxidative stress responses may have in determining possible adverse health effects, the concentrations of serum amyloid A (SAA) and high sensitivity-C reactive protein (CRP) were not significantly different between TiO2-ENM exposed and unexposed workers (65). Conversely, the serum levels of interleukin (IL)-8, IL-6, IL-1β, TNF-a, and IL-10 as possible pro-inflammatory cytokines, as well as superoxide dismutase (SOD) and malondialdehyde (MDA), as oxidative stress indicators were demonstrated to be significantly associated with occupational exposure to TiO2-ENM (65).
In this regard, a significant increase in markers of oxidative stress damage of nucleic acids and proteins in the EBC was evident in the more exposed production workers, involved in micronation, calcination and other TiO2-ENM production activities, than in the lower exposed groups of research workers and controls (69). In another study, when a panel of biomarkers of lipid oxidation was investigated in the EBC of workers exposed to TiO2-ENM during production tasks as well as in office employees of the TiO2 production plant, significant dose-dependent increases were detected compared to unexposed controls (70, 71). In a recent investigation, occupational exposure to TiO2-ENM in manufacturing and/or handling facilities resulted in significantly higher oxidative biomarkers, such as urinary 8-OHdG and EBC 8-isoprostane (38).
Such epidemiological findings are supported by extensive animal evidence concerning the deposition and bio persistence of TiO2-ENM in the pulmonary system (72–75) and the consequent development of inflammatory reactions (72, 76–82) with possible increased airway responsiveness (83) as well as acute or sub-acute airflow alterations (84). The findings of markers indicative of ROS and pulmonary inflammation (76, 78–80, 85), although reversible in some cases (86), make a suggestive case for TiO2 having pulmonary inflammogenic effects. Cardiovascular effects have been also reported in animals in which the inhalation of TiO2-ENM enhanced the phosphorylation levels of cardiac proteins (87) and impaired vasodilator response (88–91), which may be due to an increase in microvascular oxidative stress (92).
Overall, these findings support the need for future investigations primarily focused on early TiO2-ENM clinical effects on the respiratory and cardiovascular systems. Additionally, inflammatory and oxidative stress early biomarkers should be explored in depth as early biological indicators of the health impact of such xenobiotics, considering their possible predictive role of adverse outcomes. See, for example, the role of CRP as a major cardiovascular risk factor (93).
A suitable dose–response relationship has not been definitively identified. Preliminary data suggest positive relationships between levels of exposure, peculiar to job tasks in NP settings and biological changes as demonstrated by higher levels of oxidative stress biomarkers in more exposed production workers (0.13–0.76 mg/m3 particle total mass concentration; mean particle number range 0.29–2.48 × 104/cm3), compared to lower exposed research workers (0.16 mg/m3 particle total mass concentration; mean particle number 1.32 × 104/cm3) (69). These findings may give stimulus to in-depth exploration of dose–response relations with respect to biological markers of exposure such as the Ti concentration in EBC.
Cerium dioxide
Cerium dioxide (CeO2) ENM are increasingly being used in industrial and commercial applications (94, 95); however, there are no epidemiological studies of workers exposed to CeO2 ENM. Various animal inhalation and intratracheal studies show pulmonary inflammation and fibrosis (96–100), from the accumulation of CeO2 ENM in the lung tissue (101), as well as surface functionalization of the particles (96, 102). Such inflammatory responses were characterized by increased polymorphonuclear neutrophils (PMN) and lactate dehydrogenase (LDH) levels, and augmented expression of CINC-1, CINC-2, and HO-1 in bronchial lavage fluids (98, 101). Extra-pulmonary toxicity and tubular degeneration leading to coagulative necrosis in the kidney were also observed (103).
Zinc oxide
Some workers exposed to (ZnO) fume in welding and metal working operations have been shown to experience metal fume fever (104, 105). These ZnO fumes are comprised of a large proportion of NP (106). While such NP are considered combustion-derived, they may be considered “engineered” in the sense that, for welding to be effective, the appropriate specified temperature range of the materials needs to be achieved.
A study of 118 shipyard workers involved with welding and a comparison group of 45 office workers showed cardiovascular toxicity and alterations in various biomarkers [decreased cell viability, increased levels of 8-OHdG, IL-6 and nitric oxide in human coronary artery epithelial cells] (107). Exposures of the welders to particles were in the range of 5–160 nm (107). These results obtained with ZnO at the nanoscale confirmed the higher incidence of cardiovascular disease found in welders (108, 109).
Other than welding exposures, no epidemiological studies of occupational exposures to ZnO-ENM have been identified. An experimental study of ZnO fumes (2.5 mg/m3 and 5 mg/m3 for two hours) in 13 healthy non-smoking volunteers demonstrated the manifestation of fever and symptoms at the higher dose, as well as fever alone at the lower dose (110). Metal fumes generally contain NP. Exposure to ZnO at 5 mg/m3 (60 nm count median diameter; 170 nm mass median diameter) resulted in elevated plasma levels of IL-6, as well as myalgias, cough, and fatigue (110). Prior to this study, an earlier investigation of ZnO exposure in human subjects produced a metal fume fever in four human volunteers (2 hours at 5 mg/m3) (110). More recently, a dose-dependent (0.5–2 mg/m3) increase in symptoms, ie, fever, throat irritation, cough, minor respiratory symptoms, as well as flu-like symptoms, were determined after 24-hour post ZnO-ENM inhalation exposure (primary particle size of ~10 nm for 4 hours) in healthy human volunteers (111). However, the most sensitive outcomes were the concentration dependent increase in both CRP and SAA levels determined in blood 24 hours after exposure, while all concentrations of ZnO-ENM elicited significantly increases in neutrophils at the same time point (111).
In animals exposed to ZnO-ENM, in addition to pulmonary inflammation and lung injury (110, 112), researchers have found degeneration and necrosis of the myocardia after sub-chronic conditions of exposure (112). After acute and sub-chronic exposures, the following biomarkers of inflammation were detected: increased white blood cell count in the peripheral blood of exposed animals (113), transient increases in total cells and neutrophil counts, and cytokine induced neutrophil chemoattractant (CINC-1, CINC-2) and heme oxygenase-1 (HO-1) in bronchoalveolar lavage fluid (BALF) (114, 115). Co-exposure of ZnO-ENM and toluene, at their respective permissible exposure level in the paint industry, was reported to potentially produce a progressive inflammatory and fibrotic response in the alveolar tissues of the lungs of co-exposed rats (116).
Overall, the effects observed in healthy human-volunteer investigations suggest the occurrence of a systemic inflammation following ZnO-ENM exposure, which may be explained by either primary local inflammation of the respiratory tract/lung and secondary resorption of inflammatory markers or by primary systemic inflammation due to resorbed zinc ions (111). The lack of occupational epidemiological data could be addressed in future investigations aimed to define dose–response relationships, as well as possible biomarkers, like acute phase proteins CRP and SAA, which could be easily employed in biological occupational monitoring and may be indicative not only of inflammatory reactions but also possible cardiovascular events.
Carbon nanotubes and carbon nanofibers
Carbon nanotubes (CNT) and carbon nanofibers (CNF) are relatively new commercial materials. Recent epidemiological studies have specifically investigated occupational populations exposed to CNT (117–120). It is important to note that there are many different types of CNT in commerce, and these studies generally did not distinguish the specific types of CNT, other than identifying multi-walled CNT (MWCNT). Additionally, some MWCNT have residual impurities derived from the manufacturing process that can influence their reactivity and, consequently, the induced biological alterations that may function as possible biomarkers. In a pilot study, Lee et al (121) failed to find significant changes in the pulmonary function, hematology, and blood chemistry in nine MWCNT manufacturing workers compared to four office workers.
In another study to assess markers of lung inflammation, differences in FENO were observed between MWCNT-exposed workers and controls, but no differences were evident in lung function or pneumo-proteins CC16, SP-A, and SP-D (122). The reduction in FENO concentrations due to MWCNT exposure suggest that MWCNT may have an inhibitory effect on nitric oxide synthase in the airways. Also, as further respiratory effect indicators, Fatkhutdinova et al (123), found that occupational exposure to MWCNT was associated with changes in fibrotic markers. A biomarker for interstitial lung disease, KL-6, was found in increased levels in collected sputum samples in exposed workers manufacturing MWCNT compared to controls (123). Profibrotic inflammatory biomarkers, such as the cytokines IL-1β, IL-4, IL-5, IL-6, IL-8 and TNF-α were significantly higher in sputum of exposed workers than controls; and serum IL-1β, IL-4 and TNF-α were also significantly elevated in the exposed group (123).
Concerning alterations in immunological and hematological parameters, the comparison between MWCNT-exposed workers and age- and gender-matched controls showed significant upward dose-dependent trends for blood concentrations of various immunological indicators, including C-C motif ligand 20, basic fibroblast growth factor, and soluble IL-1 receptors (122). Significant decrease in neutrophils and elevation in monocytes, mean platelet volume, immature platelet fraction, and immature reticulocytes fraction were evident with increasing exposure to MWCNT (122). Beard et al (124) investigated the relationship between CNT and CNF exposure and biomarkers of fibrosis, inflammation, and oxidative stress in workers employed in manufacturing, using or distributing CNT and CNF. Variable percentages of the workforce employed in such facilities (up to 27%), had CNT and CNF present in the sputum samples as internal dose indicators (2). CNT and CNF exposure was more consistently associated with sputum fibrosis and oxidative stress biomarkers than inflammation and cardiovascular biomarkers, ie, 8-OHdG, SOD, fibrinogen, and VCAM-1 (124). In blood, positive associations were observed between exposure and biomarkers from all the above-mentioned domains, including KL-6, CRP, ICAM-1 and VCAM-1 (124). Investigating the same workforce enrolled in the Beard study (124), Schubauer-Berigan et al (117) found that the inhalable elemental carbon (EC) concentration and duration of work with CNT and CNF were positively associated with the development of respiratory allergies.
As regards cardiovascular adverse effects, a recent study (125) investigated the associations between MWCNT-exposure and biomarkers of cardiovascular risk. The authors found a significant dose-dependent upward trend in the concentration of endothelial damage marker ICAM-1 in 22 MWCNT-exposed workers, compared to age-/gender-matched unexposed controls (125). This may indicate a MWCNT-induced endothelial activation and an increased inflammatory state, which may be related to cardiovascular effects. Additionally, in another study, resting heart rate was positively related to inhalable and respirable EC concentrations (used to quantify CNT and CNF concentrations), while hematocrit counts showed a positive relationship with CNT and CNF structure counts (117).
Concerning oxidative stress reactions, MWCNT manufacturing workers had significantly higher levels of the MDA, 4-hydroxy-2-hexenal (4-HHE) and n-hexanal levels in EBC than office workers (121). Low levels of residual metal catalyst were found in air and blood, and blood molybdenum was well correlated with MDA and n-hexanal concentrations, suggesting a possible role of such metal contamination in affecting the toxicological profile of MWCNT (121). Although the study by Lee et al (121) failed to show adverse health effects, it indicated that early biomarkers of effect can be determined in the collected biological matrices. These markers were not necessary indicative of pathologic changes or early adverse effects. The markers collected in the EBC are established indicators of oxidative stress, although they may not be specific for MWCNT exposure. The use of aldehydes as dependent variables is based on their relationship with inflammation and ROS produced by activated inflammatory cells. While ROS formation is a normal physiologic process that occurs in every cell, increased levels of ROS can initiate harmful pathophysiological effects (118). Additionally, there is increasing evidence that aldelydes generated during the process of lipid peroxidation are also involved in many of the pathophysiological effects associated with oxidative stress in cells and tissues (119). Lipid peroxidation is one of the major mechanisms of ROS damage, and it occurs when oxidation of cell membranes initiates a chain reaction, which leads to the formation of aldehydes such as MDA, 4-HHE and n-hexanal such as observed in the Lee et al (121) study.
The case for risk of carcinogenicity is less clear, but may be a function of the type of CNT. In this regard, the recent IARC evaluation for CNT found that there is inadequate evidence in humans for the carcinogenicity of CNT, although there is sufficient evidence in experimental animals for the carcinogenicity of MWCNT-7 (126). For this reason, MWCNT-7 have been classified as possibly carcinogenic to humans (Group 2B), while MWCNT other than MWCNT-7 and single-walled carbon nanotubes are not classifiable as to their carcinogenicity to humans (Group 3) (126).
The whole blood gene expression profiling may also act as a tissue surrogate and may provide a powerful and an informative approach to investigate various disease conditions and identity biomarkers (127). Shvedova et al (127), in this regard, investigated the global non-coding-RNA and microRNA expression profiles in blood of exposed workers, having direct contact with MWCNT aerosol for ≥6 months and compared these profiles with those of unexposed professional and/or technical staff. Interestingly, the identified pathways and signaling networks revealed the potential for MWCNT to exert pulmonary and cardiovascular adverse effects, as well as to trigger carcinogenic outcomes in humans. Epigenetic alterations in blood cells, induced by occupational exposure to CNT, could characterize another focus for biological monitoring research. In this perspective, Ghosh et al (128) investigated whether exposure to MWCNT in the workplace may induce DNA methylation changes at the global and/or gene-specific level in some functionally important genes in peripheral blood cells. The authors identified alterations on CpG sites in the promoter regions of functionally important genes in peripheral blood cells involved in the epigenetic machinery (ie, DNMT1, HDAC4), in the DNA damage response and G1/S transition in the cell cycle (ie, ATM), as well as in oncogenic activity (ie, SKI). The detected methylation alterations might inhibit or promote the gene expression of the corresponding genes.
The biomarker findings in studies of workers exposed to CNT are consistent with pathological findings identified in studies of laboratory animals exposed via inhalation to CNT that reported pulmonary inflammation, increased collagen thickness, and robust inflammatory response with severe oxidative stress leading to fibrosis and formulation of granulomatous lesions (120, 123, 129–140). In this scenario, T helper (Th) 2-dependent type 2 immune pathways have been recognized as important drivers for the development of CNT-induced fibrosis (120). Upon stimulation, activated Th2 immune cells and type 2 cytokines interact with inflammatory and tissue repair functions to stimulate an overzealous reparative response to tissue damage, leading to organ fibrosis. Some of the animal studies also showed systemic inflammation (141), genotoxic effects (142) and an impairment in vascular smooth muscle reactivity (143), but also an immunosuppressive role of CNT exposure (144) and a carcinogenic potential due to interference with the mitotic spindle apparatus (145).
Overall, it can be concluded that at present there are no reported overt adverse effects in workers exposed to CNT. There is a consistent pattern of findings indicating that pulmonary inflammation and fibrosis could be potential outcomes in exposed populations, depending on the exposure level and duration of exposure (117, 124). Some fibrosis, inflammatory and oxidative stress biomarkers have been identified to be more strongly associated with CNT and CNF exposure (117, 124); however, to date, the lack of environmental or internal dose–exposure measurements prevents the identification of suitable dose–response relationships. Future research should clarify biological changes with respect to different exposure metrics, ie, environmental EC levels, count of CNT in sputum samples, as well as possible confounding functions due to possible metal contaminations of CNT that may bias the CNT health impact as well as biomonitoring results. Future research on global gene expression profiles as well as epigenetic effects induced by CNT may provide information to get molecular insights into the CNT-induced toxicity and pathogenesis in humans and to verify in large-scale prospective studies their validity and potential applicability as exposure and effect markers in occupationally exposed subjects (127).
Silver nanoparticles
Silver ENM (Ag-ENM) are the most common ENM in consumer products (www.nanoproject.org). Demand is growing for a wide variety of silver nanostructures, such as spheres and wires (146). A few health studies have focused on workers explicitly exposed to Ag-ENM (36, 147, 148). Two of the studies reported on the same sample of two workers, employed for seven years in silver nanomaterial manufacturing, who were exposed to 20–30-nm-sized Ag-ENM at concentrations of 0.1 and 0.4 µg/m3 respectively, in their facility (147, 148). The workers showed no significant adverse changes in their health status (147, 148). In another study, nanomaterial handling workers in 14 manufacturing facilities in Taiwan were stratified for Ag-ENM (N=6 workers); significant increases were detected in cardiovascular disease biomarkers, VCAM and IL-6, and in reduction in heart rate frequency (36).
Concerning animal investigations, toxicity in the lungs, including inflammatory reactions and histopathological alterations (149–158) were reported to be responsible for pulmonary functionality alterations (155, 159) or allergic responses (160): however, other investigations have failed to detect hematological effects, systemic alterations, and pulmonary function test changes after Ag-ENM exposure (161, 162). Histopathological changes in the kidney and liver (bile duct hyperplasia and necrosis) were identified after inhalation exposures, such as extra-pulmonary effects (155, 158, 159). Researchers have also reported changes in the expression of genes involved in xenobiotic metabolism and in the development and integrity of motor neurons; intracellular molecular patterns that regulate diverse cellular processes, including morphology, adhesion, motility; and apoptosis potentially related to neurotoxic and immunotoxic effects (163, 164).
The lack of human data relative to this widespread ENM requires further investigation on potential health effects in exposed workers and on the selection of possible biomarkers. Animal preliminary findings may be helpful to guide the verification of possible biological changes in humans.
Discussion
Understanding the effects of ENM in exposed subjects is becoming a public and occupational health priority due to the widespread application of nano-enabled products and the increased likelihood for consumer and workplace exposures. Although the number of currently available occupational field and epidemiological studies is quite limited, preliminary considerations regarding the possible health impact of ENM and biomarkers of effect can lead future investigations.
Summary of data and occupational health considerations
A summary of the findings for each of the nine high volume ENM is shown in table 2. The third column shows pathological effects in workers in epidemiological studies. That is, whether a study was identified that had significant pathologic effects related to exposure to an ENM. The fourth column reports epidemiological studies that identified a change of potential biomarkers of adverse effects.
Table 2
Summary of epidemiological and human data for engineered nanomaterial (ENM) by commercial volume. [NA=not available]
| Nanomaterial a | World commercial tonnage (tons) | Epidemiologic findings of pathologic effects in workers | Potential biomarkers of adverse effects in epidemiological studies of workers |
|---|---|---|---|
| Carbon black | 9 600 000 | Pulmonary function test alterations | Inflammatory biomarkers: white blood cell count; pro-inflammatory cytokines. |
| Synthetic amorphous silica | 1 500 000 | NA | Oxidative stress biomarkers: urinary 8-hydroxydeoxyguanosine (8-OHdG) and exhaled breath condensate (EBC); serum anti-oxidant enzymes |
| Epigenetic effects: global DNA methylation | |||
| Aluminum oxide | 200 000 | NA | NA |
| Barium titanate | 15 000 | NA | NA |
| Titanium dioxide | 10 000 | Pulmonary function test alterations; heart rate variabilities | Pulmonary disease biomarkers: serum surfactant protein-D levels; nitric oxide and leukotriene levels in EBC |
| Cardiovascular disease biomarkers: intercellular adhesion molecule 1 (ICAM-1); vascular cell adhesion protein 1, (VCAM-1); LDL | |||
| Inflammatory and oxidative stress biomarkers: pro-inflammatory cytokines, SOD and malondialdehyde serum levels; EBC markers of oxidative damage of nucleic acids, proteins, and lipids; and 8-isoprostane; urinary 8-OHdG | |||
| Cerium dioxide | 10 000 | NA | NA |
| Zinc oxide | 8 000 | Metal fume fever in healthy subjects exposed to fumes containing ZnO- ENM (not occupational) | Inflammatory biomarkers: blood C-reactive protein and serum amyloid A concentrations; neuthrophils count (not occupational data) |
| Carbon nanotubes/nanofibers | 100-3000 | NA | Pulmonary biomarkers: exhaled nitric oxide; KL-6 in sputum |
| Cardiovascular biomarkers: ICAM-1; VCAM-1 | |||
| Hematological biomarkers: blood cell count; immature cell fractions | |||
| Inflammatory and oxidative stress biomarkers: C-C motif ligand 20, basic fibroblast growth factor, and soluble interleukin (IL)-1 receptors in blood; IL-1β, IL-4, IL-5, IL-6, IL-8 and TNFβ cytokines in blood and sputum; malondialdehyde, 4-hydroxy-2-hexenal and n-hexanal levels in EBC | |||
| Epigenetic biomarkers: gene-specific DNA methylation in peripheral blood cells | |||
| Silver | 20 | NA | NA |
a Listed in table by tonnage (7).
Overall, to date there is limited evidence of adverse health effects in workers exposed to any of the most used ENM. All of the substances in table 2 except CNT have had long periods of use in some form (where the primary particle may have been <100 nm) and, hence long periods of worker exposure. Much of that exposure was to agglomerates in the micron-size range, and most of the studies did not clarify particle size, preventing the extrapolation of suitable conclusions for the toxicological profile of the nanoscale size of the chemical substances. The strongest historical findings are non-malignant respiratory disease in workers exposed to carbon black and metal fume fever in workers exposed to zinc oxide. Furthermore, explicit pathologies induced by ENM exposure have not yet been demonstrated in studies of exposed workers. The respiratory system has been reported as the primary target organ for the inflammogenic potential of both carbon-, and metal- or metal-oxide-based ENM in workplace and experimental settings, although some conflicting evidence in this regard may be due to the different physico-chemical characteristics of ENM, in terms of particle size (149), surface functionalization/coating (56, 77, 96, 102), solubility (40), particle dispersion (114), residual impurities (121) as well as level and duration of exposures (129). This is important information considering that the respiratory tract is the primary route of entry of ENM in occupational settings. Additionally, the relevance of the physico-chemical characterization of ENM in affecting their toxicological profile raises some concerns regarding the generalizability of findings obtained in occupational exposure settings where multiple ENM co-exposures may occur or where too few individuals work with a single agent preventing assessment of differences between chemicals in a meaningful way (37, 165).
Although the mechanisms for ENM-induced health effects are not fully known, the persistent inflammatory and related oxidative stress reactions induced by ENM may determine alterations in lung functionality, as reported in workers exposed to carbon black (20) or to TiO2-ENM (65). In line with these results, inflammatory responses induced by ENM exposure, and direct lung tissue damage exerted by nano-sized particle deposition and biopersistence in alveoli and bronchial walls (25, 54, 72, 101), were reported to result in fibrogenicity and increased airway hyperresponsiveness in laboratory animals (83, 97, 120, 129, 155, 159). The animal inhalation studies generally supported the human epidemiological biomarker findings and showed further significant effects for endpoints not yet seen in workers. In this regard, Th2-dependent type immune pathways have been indicated as potential triggers for the development of CNT-induced fibrosis (120). The idea that ENM may have a fibrogenic function in exposed workers may be confirmed by epidemiological findings of increased levels of pro-fibrotic markers in serum and sputum of MWCNT-handling workers (123, 124) and by RNA transcriptional analysis (127). Various findings are highly suggestive of the potential for cardiovascular dysfunction resulting from a systemic inflammatory status following pulmonary exposure (89, 90, 112, 117, 124, 125, 146, 166).
Biological monitoring implications
Overall, considering the central role of the respiratory system in the toxicokinetic and toxicodynamic profile of ENM, the EBC, which needs further validation, has been proposed as a possible biological matrix to detect biomarkers of exposure, ie, the titanium content (64) and early effect, ie, alterations in pro-inflammatory, oxidative stress indicators (66, 67, 69–71, 121, 122), as well as lung damage biomarkers (SP-D) (65). Additionally, nucleic acid, and protein and lipid oxidation biomarkers have been proposed as possible indicators of the oxidative stress reactions induced by ENM in the pulmonary system that can be assessed in EBC (69–71, 121). Moreover, considering that the persistence of inflammation and oxidative stress in the lungs may have a “systemic” impact on exposed organisms, biological monitoring investigations should consider biomarkers of systemic inflammatory and oxidative stress response in serum and changes in blood cell counts. This recommendation is supported by preliminary results demonstrating a significant association between occupational levels of exposure and increased serum concentrations of inflammatory cytokines, alterations in oxidative stress indicators, including anti-oxidant enzymes, and changes in blood cell count, ie, neutrophil reduction, and monocytes and reticulocyte increase in workers (20, 36, 65, 122–124).
Early biological alterations of blood parameters correlated to the cardiovascular functionality, ie, VCAM and ICAM concentrations as well as modifications in heart rate variability have also been explored in early human investigations on ENM toxicity with positive results for nano-silica, TiO2, and CNT (20, 65, 68, 117, 125). In line with such epidemiological data, an impaired vasodilator response due to an increase in microvascular oxidative stress or altered circulations mediators was detected in experimental animals (88–92, 167). Few human studies reported data concerning the effects of ENM exposure and CRP, a well-known cardiovascular risk factor and an easy biomarker to measure in blood samples. All these issues stress the need to examine the role of ENM exposure on the cardiovascular system, considering the possible severe implications of such alterations on the health of exposed workers, and underline the need to focus additional investigations on more easily applicable biological indicators.
Emerging scientific evidence demonstrates that environmentally-induced epigenetic alterations may play a role in the manifestation of a number of human diseases, including cancer, mental disorders, obesity, and other severe conditions (168). Although the epidemiological data concerning epigenetic changes resulting from ENM exposure are preliminary (38, 127, 128), future testing will be useful to help distinguish between adverse health effects induced by ENM exposure compared to adaptive changes. However, to initiate epigenetic toxicity monitoring for ENM exposure, it seems necessary to clarify ENM effects on the epigenome and define robust causal links between exposure, epigenetic changes and adverse phenotypic endpoints to develop improved assays to test such endpoints (169).
Additionally, considering the possible carcinogenic potential recognized for some types of ENM, ie, MWCNT-7 (126) and TiO2-ENM (129), it may be important to define early ENM biomarkers of genotoxicity. Testing this important toxicological aspect is crucial in safety assessment of new ENM compounds and products and may influence an approach to define suitable strategies for risk assessment and management in occupational settings.
Future research needs
This review revealed a need for robust longitudinal epidemiologic studies with clear exposure characterization, including particle size, extent of agglomeration, and other relevant physicochemical parameters. Of particular importance, studies that found strong evidence of adverse effects in animals should be verified/clarified in occupational field investigations. Also of concern, barium titanate, one of the ENM with significant commercial tonnage, has not been investigated for exposures or health effects; both animal and human studies should be conducted. Additionally, for cerium oxide, there is a need for epidemiological studies.
Most of the epidemiological biomarker studies on workers handling ENM are cross-sectional in nature. Among these studies, there were significant findings of biomarkers in exposed workers when compared to controls. This was true for markers of oxidative-stress with silica and TiO2 and for pulmonary, immunological and cardiovascular markers with CNT. Very few studies reported on dose–response gradients between workers in the same job profile with different exposure levels. Unfortunately, the limited data on environmental exposure levels of different ENM and biological information concerning internal doses, prevents the extrapolation of suitable dose–response relationships and the ability to define ranges of possible “dangerous exposure concentrations” (170). Having this information would allow for a reasonably accurate quantitative estimate of the occupational risks at the group and/or individual level. This seems an even more challenging issue, considering the difficulties in establishing dose-metric parameters that can calculate an ENM biologically effective dose due to the extremely variable physico-chemical characterizations of such chemicals. Future investigations should be aimed at clarifying these aspects to achieve better risk assessment and management strategies in workplaces.
Concluding remarks
As an overall conclusion, this paper takes the approach that it is not appropriate to address the question of whether there are adverse effects occurring in workers exposed in general to ENM, rather, each ENM should be investigated separately. Significant adverse indicators for specific ENM have been reported in epidemiologic and human case studies. Continuation of the use of precautionary controls for each ENM is warranted while further study of potential health effects proceeds.