Parkinson’s disease (PD) is an age-related neurodegenerative disorder, characterized by progressive motor and non-motor symptoms, which may limit patients’ daily function and impose a substantial burden on the health-related quality of life to PD patients and their caregivers (1). PD likely is caused by a combination of aging-related, genetic, and environmental factors (2). Environmental exposures, especially some aspects of lifestyle such as physical activities (PA) are modifiable and possible targets for intervention (2).
Over the past decade, animal experiments and clinical PD intervention studies suggested that intensive physical exercise may mitigate PD symptoms through effects on neuroplasticity in the nigrostriatal dopaminergic system (3–6). A number of epidemiological studies have also reported an inverse association between leisure-time physical activity and PD risk (7–11), and based on 5 prospective studies, a meta-analysis reported that being physically active reduces PD risk by 34% comparing the highest versus the lowest activity levels (12). Yet less is known about the role occupational activity may play for PD risk. Furthermore, leisure-time and work-related PA differ in their effects on mortality and risk of cardiovascular events with only leisure-time PA providing protection while some work-related PA might instead be harmful (13, 14). Here we examine both the influence of lifetime occupational and leisure-time PA at different ages on the risk of PD in a large population-based case-control study in Denmark.
Methods
Study population
From the Danish National Hospital Register, we initially identified 3508 PD cases, aged ≥35 years, who had received a primary diagnosis of idiopathic PD (code 342 in ICD-8 and code G20 in ICD-10) from one of the ten major neurological treatment centers in Denmark between 1996–2009. Of those, 2762 eligible cases were alive and available for contact, spoke Danish or English, and were able to participate in a study interview between January 2008 and December 2010. We excluded 179 (6%) patients, whose medical records did not confirm PD in a screening review before the interview. Of the remaining 2583 cases we contacted, 2086 (81%) consented to participate and medical records were available for 2066 (99%). A detailed medical record review by trained health professionals supervised by a movement disorder specialist allowed us to exclude cases of parkinsonism. In this review, we applied standard diagnostic criteria from the United Kingdom Brain Bank (15) and criteria published by Gelb et al (16), and defined an idiopathic PD diagnosis as (i) having ≥2 of 4 cardinal symptoms, including resting tremor, bradykinesia, rigidity and asymmetrical onset; (ii) positive response to antiparkinsonian medication; 3) no atypical features, such as dementia before development of cardinal symptoms, early falls, severe symptomatic dysautonomia, rapid progression of the disease, sudden onset of symptoms, supranuclear gaze palsy, hallucinations unrelated to medication, freezing phenomena, and Babinsky reflex; and (iv) no sign of a differential diagnosis (eg, cerebrovascular disease). After review, we excluded 238 cases and confirmed a total of 1828 (88.5%) participants with idiopathic PD.
For each of the initially contacted 2583 PD patients, 5–10 potential gender and year of birth matched control subjects without a diagnosis of PD at or prior to the index date (date of first hospital contact for PD for the matched case) were randomly selected from the Danish Central Population Register. We contacted controls in a random order until one consented to participate; for 121 PD cases, all controls refused to participate, and new controls were selected from a pool of extra controls sampled. A total of 3626 eligible controls were contacted and 1909 (53%) agreed to participate and completed an interview.
We further excluded 14 cases and 22 controls who had received a diagnosis of dementia (ICD-8 codes 290.09–290.19 or 293.09; ICD-10 codes F00–03, F05.1 or G30) or cerebrovascular disease (ICD-8 codes 430–438; ICD-10 codes I60–69, G45 or G46) between 1977 (when the hospital registry began) and up to three years before index date; one case was excluded due to unknown diagnosis date. Lastly, to ensure that we had sufficient information for lifetime occupational PA, we also excluded those who did not provide a complete work-related history, leaving 1640 cases and 1715 controls for analysis.
Physical activity assessment
Trained interviewers obtained information on work-related and leisure-time PA, medical history, lifestyle factors, and family history of PD in a structured telephone interview between January 2008 and December 2010. Mailed paper questionnaires were provided to some participants either because of physical limitations or personal preference (N=618), and the proportion of participants who returned a completed questionnaire was similar for cases and controls. For each participant, we collected a full employment history starting at or after 14 years of age and ending at the index date. A code for each job was assigned according to the International Standard Classification of Occupations 1968 (ISCO68) of the International Labor Office (ILO, 1969). We created a job exposure matrix (JEM) to estimate occupational PA blinded to case status. Each job code was assigned a metabolic equivalent (MET) value based on position (sitting, standing, walking, or heavy physical demands) and intensity (light, moderate or strenuous) as developed by Tudor-Locke et al (17) (supplementary table A, www.sjweh.fi/index.php?page=data-repository). Lifetime cumulative occupational PA was calculated by multiplying the length of the reported years of work with the MET values assigned to all jobs. Participants were also asked to report how often they engaged in strenuous leisure-time PA (defined as an activity that makes a person breathe hard, such as jogging, aerobics, weight lifting, basketball) during three age periods: 15–25, 25–50, ≥50 years of age. Four possible answers were allowed: 0, 1–4, 5–10, ≥10 hours per week.
The Danish Data Protection Agency (no. 2002–41–2112) and the University of California, Los Angeles (UCLA), Institutional Review Board for human subjects approved the data collection and procedures described in this study. All participants granted written informed consent.
Statistical analysis
Unconditional logistic regression analyses were performed using SAS software version 9.3 (SAS Institute, Inc, Cary, NC, USA), with adjustment for gender, age (at index date), smoking (ever/never), coffee consumption [cup years in tertile; 0–122, 122–220, >220), level of education (basic school, high school (7–12 years); vocational (10–12 years); and higher education (>13 years)], and family history of idiopathic PD (IPD) (first degree relatives suffering from IPD yes/no). We reported odds ratios (OR), 95% confidence intervals (95% CI), and P-values for trend based on the median of each exposure category (2-tailed significance level at α=0.05). Analyses were conducted separately for occupational and leisure-time PA for all subjects and stratified by gender. We categorized cumulative occupational PA into quartiles (Q1, Q2, Q3 and Q4) based on the distribution in controls, with the first quartile (lowest level of physical activity) representing the reference group. In our analyses, we used the entire working life as well as cumulative occupational PA prior to the age of 30 and 50 years to assess the potential age-specific effects. For leisure-time PA, those who did not engage in strenuous activities formed our reference group, and we compared them with participants who reported 1–4 and ≥5 hour/week in each age period. We also included both activity measures (work and leisure-time) in the same model to mutually adjust our estimates. In addition, to explore whether changes in leisure-time PA levels throughout life were associated with PD risk, we classified levels into low/high for each age period. At 15–25 years, we considered 0 and 1–4 hours/week as low, and everyone else as having high activity; in all other age periods, only those who never participated in strenuous activities were considered low and everyone else as high. We then compared those who reported a consistently high activity and those who reported either low-high or high-low trajectories to those who reported consistently low activity throughout their life. We further created a new variable to assess the combined exposure to both PA types at <25 and 25–50 years of age. We distinguished each age period and assessed combinations of low versus high levels of occupational PA (cutoff: median) and hours of strenuous activities performed during leisure time (0, 1–4, ≥5 hours/week).
We conducted additional sensitivity analyses (i) including only incident cases (those diagnosed between 2006–2009) or (ii) using exposure lagging and discounting activities within a 10-year period prior to the index date for the entire lifetime occupational PA measures to assess the potential influence of PA changes in the prolonged preclinical phase of PD.
Results
Study participants were on average 68 years of age at the time of interview and PD cases were 60.8 years at the time of diagnosis or first symptom onset (table 1). Years of education and duration of years cases and controls had spent working were very similar, though men reported more working years than women. More cases were never smokers and less likely heavy coffee drinkers, and as expected, a family history of PD was more prevalent among cases than controls.
Table 1
Demographic characteristics of the study population by Parkinson’s disease status.
In multivariate-adjusted models for men and women combined, participants in the highest (≥125.8 MET-year) and second highest (94.5–125.8 MET-year) categories of entire lifetime occupational PA had a 19% lower PD risks than participants in the lowest (<70 MET-year); a marginally significant dose–response with increasing levels of occupational PA was observed (P for trend = 0.06) (table 2). While neither overall nor gender-specific inverse association was found in lagged analyses that excluded occupational PA 10 years prior to PD diagnosis (supplementary table B, www.sjweh.fi/index.php?page=data-repository), we did observe this association when we limited analyses to work activities participants had engaged when <30 years of age. Gender-stratified analyses showed that risk was lower only in the highest category of occupational activities in women engaged in occupational PA in young and middle adulthood; these negative associations remain unchanged when we further limited our analyses to incident cases only (diagnosed between 2006–2009; supplementary table C, www.sjweh.fi/index.php?page=data-repository).
Table 2
Multivariable-adjusted odds ratios (ORadj) for Parkinson’s disease and occupational physical activity. [MET=metabolic equivalent; 95%CI=95% confidence interval.]
| MET-year | All | Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Case/Control | ORadja | 95% CI | P-trendb | Case/Control | ORadja | 95% CI | P-trendb | Case/Control | ORadja | 95% CI | P-trendb | |
| Entire work-life | ||||||||||||
| <70.0 | 450/427 | 1.00 | . | 231/204 | 1.00 | . | 219/223 | 1.00 | . | |||
| 70.0–94.5 | 415/424 | 0.90 | 0.74–1.10 | 245/256 | 0.85 | 0.65–1.13 | 170/168 | 1.00 | 0.74–1.34 | |||
| 94.5–125.8 | 392/434 | 0.81 | 0.66–1.00 | 245/271 | 0.78 | 0.59–1.04 | 147/163 | 0.87 | 0.64–1.18 | |||
| ≥125.8 | 383/430 | 0.81 | 0.65–1.01 | 0.06 | 263/309 | 0.79 | 0.58–1.06 | 0.16 | 120/121 | 0.89 | 0.63–1.26 | 0.39 |
| Prior to age 50 years | ||||||||||||
| <57.5 | 451/413 | 1.00 | . | 231/199 | 1.00 | . | 220/214 | 1.00 | . | |||
| 57.5–75.5 | 395/437 | 0.84 | 0.69–1.02 | 235/267 | 0.80 | 0.61–1.05 | 160/170 | 0.95 | 0.71–1.28 | |||
| 75.5–100.9 | 408/438 | 0.83 | 0.68–1.01 | 244/273 | 0.81 | 0.62–1.07 | 164/165 | 0.92 | 0.68–1.23 | |||
| ≥100.9 | 386/427 | 0.83 | 0.68–1.03 | 0.13 | 274/301 | 0.90 | 0.68–1.20 | 0.88 | 112/126 | 0.75 | 0.54–1.06 | 0.12 |
| Prior to age 30 years | ||||||||||||
| <21.3 | 499/463 | 1.00 | . | 267/243 | 1.00 | . | 232/220 | 1.00 | . | |||
| 21.3–33.5 | 421/411 | 0.93 | 0.77–1.13 | 227/219 | 0.97 | 0.74–1.28 | 194/192 | 0.94 | 0.71–1.24 | |||
| 33.5–45.5 | 342/419 | 0.76 | 0.62–0.93 | 212/270 | 0.80 | 0.61–1.06 | 130/149 | 0.77 | 0.57–1.05 | |||
| ≥45.5 | 378/422 | 0.84 | 0.68–1.03 | 0.04 | 278/308 | 0.91 | 0.69–1.19 | 0.38 | 100/114 | 0.76 | 0.54–1.08 | 0.06 |
Leisure-time PA was associated with a 25% lower risk of PD among those who reported engaging in ≥5 hours of strenuous activity per week in young adulthood (15–25 years) (table 3), but gender-stratified analyses showed that these negative associations were only due to activities reported by men. In contrast, for women, we estimated inverse associations with PD only at high levels of leisure-time PA after age 50. These patterns were also observed among incident cases only (supplementary table D, www.sjweh.fi/index.php?page=data-repository). In terms of changes in leisure-time PA over the lifetime, those who remained highly active throughout their life from young to older adulthood were at lowest risk of PD (OR=0.72, 95% CI 0.59–0.89), followed by those with either a high-low or a low-high trajectory in comparison with those who were consistently less active (supplementary figure A, www.sjweh.fi/index.php?page=data-repository). Yet, this trend was only observed among men but not women.
Table 3
Multivariable–adjusted odds ratios (ORadj) for Parkinson’s disease and leisure-time physical activity (PA). [95% CI=95% confidence interval.]
| Age (years) and hours/week of leisure-time PA | All | Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Case/control | ORadja | 95% CI | P–trendb | Case/control | ORadja | 95% CI | P–trendb | Case/control | ORadja | 95% CI | P–trendb | |
| 15–25 (hours/week) | ||||||||||||
| 0 | 523/510 | 1.00 | . | 298/266 | 1.00 | . | 225/244 | 1.00 | . | |||
| 1–4 | 731/691 | 0.99 | 0.84–1.17 | 377/355 | 0.90 | 0.71–1.13 | 354/336 | 1.11 | 0.87–1.41 | |||
| ≥5 | 434/534 | 0.75 | 0.62–0.90 | 0.003 | 324/419 | 0.63 | 0.50–0.79 | <0.001 | 110/115 | 1.03 | 0.87–1.44 | 0.69 |
| 25–50 (hours/week) | ||||||||||||
| 0 | 690/725 | 1.00 | . | 382/414 | 1.00 | . | 308/311 | 1.00 | . | |||
| 1–4 | 711/723 | 0.99 | 0.85–1.15 | 396/405 | 0.99 | 0.81–1.22 | 315/318 | 0.96 | 0.87–1.21 | |||
| ≥5 | 272/283 | 0.96 | 0.78–1.18 | 0.69 | 210/215 | 0.99 | 0.77–1.26 | 0.92 | 62/68 | 0.86 | 0.87–1.28 | 0.49 |
| ≥50 (hours/week) | ||||||||||||
| 0 | 795/798 | 1.00 | . | 469/500 | 1.00 | . | 326/298 | 1.00 | . | |||
| 1–4 | 640/721 | 0.84 | 0.73–0.98 | 349/383 | 0.91 | 0.75–1.12 | 291/338 | 0.75 | 0.87–0.94 | |||
| ≥5 | 169/175 | 0.91 | 0.72–1.16 | 0.10 | 123/115 | 1.06 | 0.79–1.42 | 0.93 | 46/60 | 0.65 | 0.87–0.99 | 0.01 |
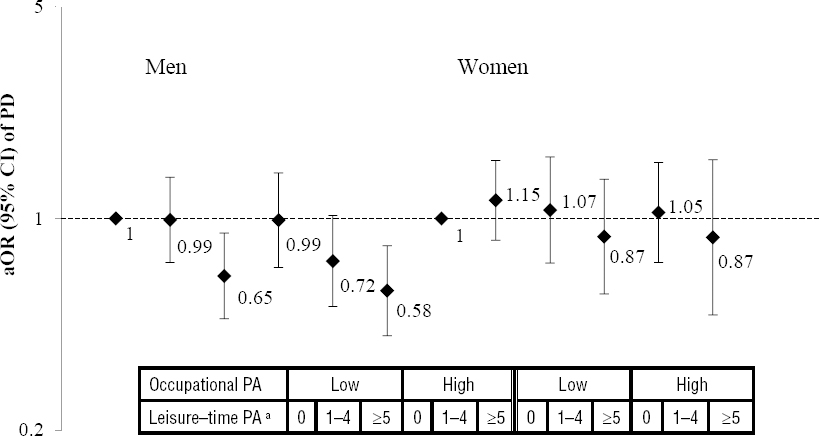
The relationship of combined occupational and leisure-time PA before age 25 and PD risk was evaluated in all participants (supplementary table E, www.sjweh.fi/index.php?page=data-repository) and stratified by gender (figure 1). After adjustment for confounders, participants with high levels of both work and leisure-time activity (≥5 hour/week) before age 25 had a 34% lower risk of PD (OR 0.66, 95% CI 0.50–0.87). At low levels of occupational PA, we estimated a borderline decrease in PD risk among those engaged in ≥5 hour per week of strenuous activity at leisure-time (OR 0.80, 95% CI 0.62–1.02), however, the inverse association for young adults was mainly due to men’s activities. We observed no association between combined PA in the 25–50 year age group and risk of PD (results not shown).
Figure 1
Multivariable-adjusted odds ratios (aOR) with 95% confidence intervals (95% CI) of Parkinson’s disease (PD) according to the combination of occupational and leisure-time physical activity prior to age 25 years, stratified by gender. The analysis is adjusted for sex, education, smoking, coffee consumption, age, index age, and family history of PD. aLeisure-time physical activity (PA) is categorised in hours/week.
Discussion
In this large population-based case–control study, we observed age-period and gender-specific influences of leisure-time and occupational PA on PD risk. Specifically, strenuous leisure-time PA in young adulthood was inversely associated with PD risk for Danish men but not women, while Danish women seemed protected by young and middle adult occupational PA only. Interestingly, after age 50, leisure-time PA made some difference for PD risk among Danish women but not men who – on the other hand – were protected if they remained highly active throughout their life. These results did not change when we restricted to incident cases only or lagged exposures (ie, excluded activities in the last ten years prior to PD diagnosis).
Several biological mechanisms in support of a protective effect of PA on PD have been proposed, including an increase of neurotrophic factors, downregulation of the dopamine transporter and reversing of a reduction of dopamine D2 receptor in the dorsal striatum (3, 18). In rodent models of PD, animals forced to exercise either before or after neurotoxin administration retained more dopamine neurons and dopaminergic terminals and exhibited better motor performance compared with neurotoxin-treated immobilized animals (3, 18). There are also a few human studies where early stage PD patients underwent 8-weeks of high-intensity treadmill training which increased corticomotor excitability and dopamine D2 receptor binding potential along with improved clinical motor function (5, 6).
Leisure-time PA has also been consistently negatively associated with risk of PD onset (7, 11, 8, 10, 9). However, few studies assessed such activities during several age periods or attempted to address reverse causation. Our findings add additional insights to this discussion by exploring PA during different age periods and assessing both at work and leisure-time activities. Our results are in line with previous studies that found PA during adulthood (ages of 25–50 years) not to be associated with PD risk (8, 11); in fact inverse associations were observed only with PA in very young adulthood (11, 7) or in senior years prior to PD onset (7, 11, 8, 10, 9), the latter possibly being explained by reverse causation. With regard to change in leisure-time PA from young to middle-aged adulthood, our evaluation of activity trajectories supports previous findings that individuals who consistently and frequently participate in strenuous PA throughout their lifetime were at lowest risk of PD (9, 11). Our large sample size also allowed us to stratify by gender. We found that prominent inverse associations with strenuous leisure-time activity were only observed before age 25 for men while women seemed only protected by activities after age 50. Interestingly, two previous US studies found inverse associations for both young men and women (11, 7), which might suggest a difference in leisure-time activity among Danish and US women of the generation currently included in PD studies. It is possible that US women engaged in higher levels of leisure-time sports activities since US women began to form athletic clubs after 1870 and competitive sports for college women have been common since the early 1900s (19). In contrast, Danish women have not been encouraged to be as active as men in terms of sports-related exercise until the 1980s, when only 11% of Danish women reported that they regularly played sport as compared to 21% of men in 1964 (20).
So far only one Swedish and two US studies assessed the effects of job-related activities on PD risk. While no association was found in Sweden and an agricultural population in central California (11, 12), a study among US retired people suggested a reduced PD risk for those who reported routinely engaging in physically demanding work (ie, lifting or doing heavy work), compared with those reporting to mostly sitting at work (9). However, the later study only collected occupational activities at baseline (enrollment ages were 50–71 years) and followed participants for ten years at the most. Due to the long preclinical phase of PD, this baseline assessment of PA could have already been affected by prodromal PD symptoms causing a reduction of PA and thus these associations may have been due to reverse causation. Interestingly, when we examined the association between combined PA and risk of PD, men – but not women – with high PA levels during leisure time and working hours before age 25 had the lowest PD risk. Gender-specific associations between occupational PA and all-cause mortality have previously being reported (13, 14, 21). For example, a Danish study reported an increased risk for all-cause mortality from the high occupational activity only among male workers whereas another study found a negative association instead among Danish adult females, but not for males (14, 21). Though gender-specific mechanisms between work-related PA and PD are hard to explain, gender differences in risk factors for PD have been reported previously (2) and the physiological differences between genders for similarly physically demanding work may also play a role.
The strengths of our study are the very large number of PD cases we assessed with a detailed review of medical records supervised by a movement disorder specialist that reduced the chance of misdiagnosis, and a study population identified from Denmark’s reliable national registers. Additionally, we used a JEM to assess occupational PA with expert raters blinded to case status avoiding recall bias in our case–control design. Nevertheless, our JEM approach is prone to non-differential misclassification of occupational activities because we assume that job titles reflect the same level of physical activity (ie, MET value) for all workers when within-job variability is likely and this may lead to underestimating associations. Another limitation is that “housewife/homemaker” is not considered a job in our study. Those who were full-time housewives/homemakers might have been physically very active especially at younger ages while taking care of children, and our study did not take this into account. In terms of assessing lifetime leisure-time PA, previous studies have shown that length of the recall period is inversely associated with the level of recall accuracy (22, 23), but strenuous-intensity activity was more precisely recalled than light or moderate activities (22, 24). In addition, the self-reported PA may be affected by recall problems in our case–control design. While it is generally assumed that cases spent more effort to recall exposures, it is possible that PD cases underreport lifetime activity levels due to a less active lifestyle after years of suffering from PD, and thus the bias may be in either direction. Because of our recruitment and interview schedule, selective survivorship is a possibility. All PD patients and birth year matched controls were identified between 1996 and 2009, but interviews started in January 2008, which required everyone to have survived at the most for 12 years before being interviewed (deceased PD cases or controls were not included). To assess the influence of survival bias we therefore conducted sensitivity analyses limiting our population to incident cases; our results for young adulthood leisure-time PA in men and occupational PA in women did not change but the inverse association between entire lifetime occupational PA and risk of PD in both men and women disappeared. Moreover, excluding PD cases diagnosed prior to age 60 did not substantially change physical activity estimates or conclusions (results not shown). We cannot exclude reverse causation as an explanation; however, our results for young adulthood activities would require us to assume that physical activity at work or in leisure time is influenced by PD already at a very early adult age.
Concluding remarks
The present study suggests that the physical activity that young women and men engage in matters in determining PD risk, but also that the type of activity they engage in depends on gender roles. For women, only work-related activity in young and middle adulthood seemed to protect against PD, while among men only leisure-time activity was inversely related to PD except when occupational and leisure-time PA were both high among young men. This may be explained by differences in the types of physical demands in male and female occupations. Men who have the opportunity and ability to engage in exercise and sports even when their work is physically demanding may hold special types of job that selects for the physically fittest men.



