The prevalence of odd work hours has increased during the last decades in Europe and many other countries (1). According to the last survey in Europe, 17% and 10% of workers do shift and night work, respectively. In addition, 53% work at the weekend, and 20% work on call (2). Shift work is associated with many health problems like sleep disorders, fatigue, accidents, cardiovascular disease, gastrointestinal disorders, and cancer (3). In 2007, the International Agency for Research on Cancer (IARC) (4) concluded that “shiftwork that involves circadian disruption is probably carcinogenic to humans”. The IARC review further stated that the association between shift work and breast cancer was supported by sufficient evidence among experimental animals but only limited evidence in epidemiological studies on humans. Regarding cancer, shift work has been associated with breast cancer (5, 6, 7), prostate cancer (8), endometrial cancer (9), colorectal cancer (10), and Non-Hodgkin’s lymphoma (11). The most extensively studied cancer in this context is female breast cancer. In a recent review, nine epidemiological studies were reported, of which six were positive (12). Since then, an additional four studies have been published (13–16), three of which were positive.
Nearly all living organisms have adapted to the 24-hour alternating pattern of days and nights. This endogenous, circadian rhythm is entrained to the individual’s environment by light stimulus through the eyes. The master clock, which is located in a paired suprachiasmatic nucleus (SCN) in the hypothalamus, helps the organism to adjust to the environmental 24-hour cycles.
Present in all organs of the human body, clock cells have the ability to create an endogenous biological rhythm. The peripheral clock cells, located outside the hypothalamus are influenced by the SCN clock, but they are also entrained by the timing of food intake. The clock cells have specific clock genes that create a circadian rhythm by means of a cyclic production of specific proteins.
Clock genes take part in crucial functions of cell biology, eg, cell proliferation (17), DNA repair (18), and apoptosis (19). The cell division is temporally regulated, and this rhythmic control affects the rate of the DNA replication (20). In experimental studies on rodents, it has been shown that eradication of the SCN leads to disturbance of the circadian rhythm and increases the growth of malignant tumors (21). Exposure to light at night (LAN) has a similar effect on the circadian system (22). Animal experiments have demonstrated that LAN accelerates the growth of breast cancer cells (23). A recent epidemiological study on women with experience of long-term night shift work showed that shift work was associated with epigenetic changes of the clock genes CLOCK and CRY (24). The same epigenetic changes have been observed in breast cancer case–control studies (25).
Melatonin is a chronobiotic hormone produced by the pineal gland that transmits information on environmental darkness and lightness to the body (26). Melatonin has anticarcinogenic actions that are mediated by a number of mechanisms, including antioxidant and antimitotic activity (26, 27, 28). In addition, melatonin has antiestrogenic properties. Since estrogen promotes growth of breast cancer cells, deficiency of melatonin stimulates their growth (27). Melatonin is manufactured and secreted mainly during the night. If the individual is exposed to LAN, the production of melatonin decreases acutely. Depending on the time when LAN occurs, it can also shift the phase of melatonin in blood.
Erren & Reiter (29) have suggested the following definition of circadian disruption or chronodisruption: “a breakdown of phasing internal biological systems appropriately relative to the external, ie, environmental changes, which leads to chronobiological disorders” (29). They also suggest that the best markers today of chronodisruption are melatonin or its metabolites in saliva, urine, and blood, and observational surrogates such as work at unusual times and/or unusual sleep patterns. As concerns shift work, shifts including night work are supposed to be the strongest chronodisruptor. The reason for this is that night work apparently is associated with exposure to LAN. However, studies on people working night shifts have not yielded consistent results with respect to melatonin levels (30–33). Maybe, the mean level of melatonin is not as important as the amplitude of the curve or the phase shift. Exposure to LAN will shift the melatonin acrophase forward if the subject is exposed in the late evening or early night. If the exposure is present in the early morning, the shift of the acrophase will be backward. Thus, it is a possible that not only night shifts but also late evening or early morning shifts can have chronodisruptive properties. We are aware of only two published studies reporting the risk of breast cancer in relation to shift work without night shifts. None of those showed increased risk among the shift workers (34, 15). There is a need for more studies comparing the risk of breast cancer in relation to different shift systems, also systems that do not include night work. The aim of the present study was to compare the risk of breast cancer among women with day work and shift work with and without night shifts.
Methods
The data were obtained from the WOLF (Work, Lipids, and Fibrinogen) occupational cohort study that included subjects who were employed in different public and private companies (35). The baseline study was first carried out in Stockholm, Sweden, from 1992–1995 (WOLFS, N=5698). In order to include more subjects, who worked in blue-collar jobs, a new data collection was carried out in two counties in the north of Sweden from 1996–1997 (WOLFN, N=4718). All subjects were employed at baseline (aged 19–70 years) and worked in 60 different companies. The major branches were the pharmaceutical industry, transportation, public administration, telecommunication, sales work, schools, the paper and pulp industry, banks, offices, and the mechanical industry. The overall participation rate was around 80%. The subjects who participated in the 1996–1997 data collection were invited to a follow-up examination, which was performed during 2000–2003 (WOLFF, N=5433). Of those, 3630 were re-examined, and 1803 were recruited for the first time. In the present analysis, we included all women who entered the cohort in 1992–1995, 1996–1997, and 2000–2003, in total 4087 employees. Among those, it was possible to classify exposure to shift and day work among 4036 women, comprising the analytic sample for this study.
At baseline, the participants answered a questionnaire (at home) and were examined by nurses at 33 occupational health units. The questionnaire was extensive and contained questions about work tasks, occupation, work hours, education, work environment, stress, behavioral factors, family, physical activity, sleep, health, pharmaceuticals, hereditary issues, and foreign extraction. The medical examination included measurement of height, weight, abdominal circumference, hip circumference, and blood pressure. The nurses, who performed the examination, were trained beforehand to ensure standard procedure.
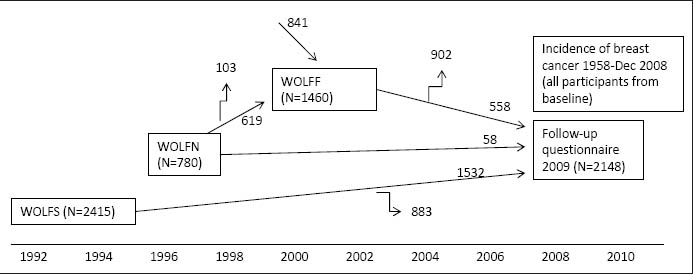
A new questionnaire was sent to all participants in 2009. In total, 2148 women answered the questionnaire in 2009 (WOLFU). Figure 1 shows the flow chart in the present study.
Figure 1
A flowchart for description of the WOLF cohort. WOLFS=WOLF Stockholm; WOLFN=WOLF Norrland; WOLFF=follow-up of WOLFN and new recuitment to WOLFN.
Shift work
The two following questions were included in the WOLFS (1992–1995) and WOLFN (1996–1997) questionnaires: (i) “Do you work shifts?” Possible responses: (a) no; (b) 2 shifts; (c) 3 shifts with continuous operation; (d) according to a rota (irregular scheduling or working hours round the clock and over the whole week according to a particular work schedule; (e) other type of shift. (ii) “How many hours do you normally work per week including overtime, and how are these hours distributed on average?” Possible responses: (a) day work (06:00–18:00 hours), number of hours=xx; (b) evening work (18:00–22:00 hours), number of hours=xx; (c) night work (22:00–06:00 hours), number of hours=xx.
In the WOLFF questionnaire (2000–2003), the questions were similar but the response options to question (i) were changed to: (a) no; (b) 2 shifts; (c) 3 shifts with continuous operation; (d) according to a rota without night shifts (irregular scheduling or working hours round the clock and over the whole week according to a particular work schedule); (e) according to a rota with night shifts; (f) permanent night work; and (g) other type of shift.
In addition, the WOLFF questionnaire asked if the respondent had changed shift system during the last five years – either from shift to day or vice versa.
In the WOLFU questionnaire in 2009, the following questions were asked: (i) “Which option is best suited in terms of your current working hours? If you are not employed right now we want you to think of the time just before you stopped working?” (a) day work (about 06:00–18:00 hours); (b) evening work (about 18:00–22:00 hours); (c) night work (about 18:00–06:00 hours); (d) shift work, not night; (e) shift work, including night; (f) according to a rota (ie, working hours according to a particular work schedule), not night; (g) according to a rota (ie, working hours according to a particular work schedule), including night; (h) other working hours. (ii) “Over how many years of your career have you worked shifts? If you have never worked shifts, enter 0 years.” (iii) “How many years have included night work? If you have never worked shifts with night work, indicate the last year you worked shifts, and enter 0 years.”
In order to categorize the participants in three groups (ie, day work and shift work with and without night shifts), we used data from baseline, follow-up in 2000–2003 (WOLFF), and follow-up in 2009 (WOLFU). If data indicated day work on all occasions when the subject participated, she was regarded as a day worker. If data indicated shift work without night work on ≥1 occasion, and day work for the rest, the participant was defined as a worker with shift work without night work. If the data indicated shift work with night work on ≥1 occasion, and day work or shift work without night for the rest, the participant was regarded as a worker with night shift work. Information on shift/day work was obtained from 1459 participants from baseline only. In total, 2148 subjects participated in baseline and WOLFU, and 429 subjects participated on all three occasions (WOLFN or WOLFS, WOLFF and WOLFU).
Breast cancer
Data on cancer incidence were obtained from the Swedish cancer registry from its establishment in 1958 to 2008. Reporting is mandatory and covers the total population (36). It includes individual data on personal identification number, sex, place of residence, site of tumor, histological type, basis and date of diagnosis, date and cause of death, date of migration, and whether a patient was registered as a resident in Sweden at the end of a specific year. However, cases without a cancer notification, but reported to the Cause of Death Register, are not included. Breast cancer codes according ICD-7 (170) or ICD-10 (C50) were used to define incident breast cancer cases. The date and causes of death were obtained from the Swedish death registry between 1992–2008. Women who developed breast cancer between 1958 and baseline were excluded from the analyses (N=10). All deceased women with breast cancer as an underlying cause in the death certificate (N=8), were included in the Swedish cancer registry in the present study.
Covariates
Information on the following potential confounders was collected at baseline: anthropometric variables (height, weight, waist and hip circumference) were measured, and information about educational level, number of children, smoking, menopausal status, oral contraceptive use, hormones other than contraceptives, alcohol intake, and educational level was obtained from the questionnaire. Body mass index (BMI; weight in kilograms/height in square meters), and waist–hip ratio (waist/hip in centimeters) were calculated based on the anthropometric measures.
Statistical analysis
All statistical analyses were performed using SPSS/Windows, version 17.0 (SPSS Inc, Chicago, IL, USA). The results are expressed as percentages for dichotomous variables and means for continuous variables. Chi-square test was used for categorical variables. Number of children was tested by Mann Whitney U-test (due to a skewed distribution). Two-tailed tests were used. Cox regression analysis was used for multivariable analysis. Hazard ratios (HR) are expressed with 95% confidence intervals (95% CI). Attained age was used as time variable (37). When restricting the population to <60 years of age, censoring was done for time of death, time of diagnosis or when the subject reached age 60, whichever came first. The working time variable was categorized into day work and shift work with and without night work. Day work was used as reference. The following were potential confounding variables from baseline: (i) body mass index (BMI) (continuous); (ii) waist-hip ratio (continuous); (iii) educational level dichotomized in two ways: educ1 (coded 1 for university or college, coded 0 for lower than university/college education), educ2 (coded 1 for high school or higher level, coded 0 for lower level than high school); (iv) current smoking (current versus not current smoker); (v) ever smoker (ever versus never smoker); (vi) menopausal status, and (v) treatment with hormones other than oral contraceptives. The variable “number of children” was categorized into four categories: 0, 1, 2, ≥3 children. Alcohol intake was based on the question “On average how many alcoholic beverages have you imbibed during the past 12 months? Indicate with a cross how often and specify how much.” A new variable was calculated that was dichotomized. High level of alcohol intake was defined as consumption of alcoholic beverages (beer, wine or liquor) on average ≥6 times per week. Low level was defined as less than that. All variables were not kept in the main model because they only marginally influenced the HR. Therefore, the final model included only exposure to day/shift work, number of children and alcohol intake.
Results
The mean follow-up time (time from baseline to censorship) was 12.4 years (range 0.2–16.1). When censoring participants for time of death, time of diagnosis, or 31 December 2008, the total number of person-years in the cohort was 49 973. Breast cancer was diagnosed among 97 women between baseline and follow-up. Three of these were excluded because data on the shift work variable were missing.
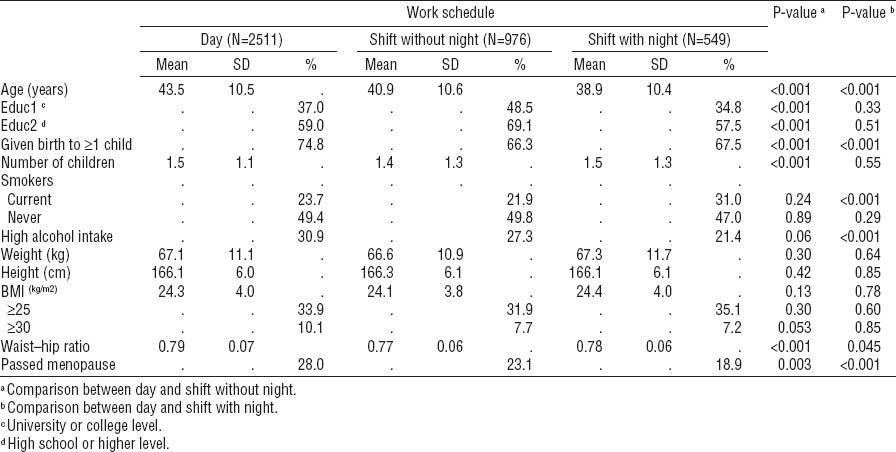
Table 1 shows characteristics of the three groups at baseline. The two groups of shift workers were younger than the day workers. Nulliparity was more common among shift workers. Smoking was more prevalent among night workers. No significant differences were found regarding BMI, but waist–hip ratio tended to be lower in the two groups of shift workers. Compared to shift workers, a higher proportion of day workers had passed menopause. High alcohol consumption was more prevalent among day workers.
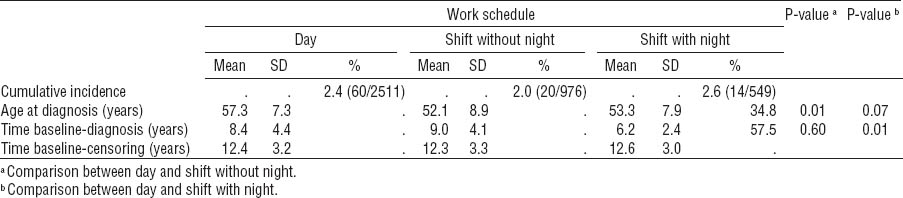
The cumulative incidence in the three groups is shown in table 2. Age at diagnosis was lower in the two groups with shift work, compared with those in the day working group. Shift workers with night work tended to have a shorter time from baseline to diagnosis than day workers.
Table 2
Cumulative incidence of breast cancer, age at diagnosis, and time from baseline to diagnosis. [SD=standard deviation.]
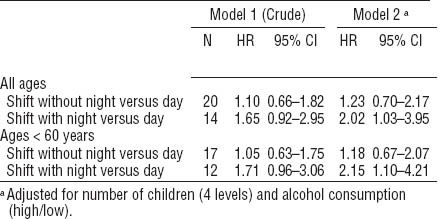
Results of the Cox regression analysis showed increased HR for shift workers with night work compared with day workers (table 3). When analysis was restricted to those women <60 years of age, the HR increased further for shifts with night work.
Table 3
Hazard ratio of breast cancer cases. Results of Cox regression analysis. [HR=hazard ratio; 95% CI=95% confidence interval.]
It was possible to calculate the exposure time for night work using responses from those who answered the WOLFU questionnaire [N=341, mean 9.39 years, standard deviation (SD) 9.53]. It was also possible to compare the information provided in WOLFU with baseline data. Of those who reported that they had had no experience of shift work (with or without night work) in WOLFU, 22% had reported such experience at baseline. Of those who reported they never had worked shift with night shifts in the WOLFU questionnaire, 2% reported night shifts at baseline.
Discussion
This study indicates that shift work with night shifts is associated with an increased risk of breast cancer. This finding is consistent with a number of published epidemiological studies. The adjusted risk was 2.02, which is comparable with that of Davis et al (6) [odds ratio (OR) 2.3, duration of exposure >4.6 years], Lie et al (7) (OR 2.21, duration of exposure ≥30 years), and Pesch et al (38) (OR 2.48, duration of exposure ≥20 yrs). Tynes (39) reported higher risk [risk ratio (RR) 3.2, duration of exposure <3.2 years and age >50 years; risk 4.3, duration of exposure >3.2 years and age >50 years]. Hansen (40) found a slightly lower risk compared with our results (OR 1.7, duration of exposure >6 years of night work) and Schernhammer (41) (RR 1.79, duration of exposure ≥20 years). In our study, it was not possible to obtain data on the duration of lifetime exposure for all participants, only for those who answered the questionnaire in 2009.
Our results showed that shifts without night work were associated with a HR of 1.23, which is not statistically significant. Hansen et al (15) did not find any increased risk [OR 0.9, 95% CI 0.4–1.9] in a Danish case–control study on nurses who worked evening shifts (the shift started in the afternoon and ended at 23:00 or 24:00 hours). A case–control study from the USA showed an OR of 1.08 (95% CI 0.81–1.44) among evening shift workers (34). The evening shift, however, was a mix of evening and night work, since it started in the afternoon and could end as late as 02:00 hours.
When considering only cumulative incidence, there were only marginal differences between the three work schedules. However, the shift workers with night shifts were younger at inclusion and diagnosed with breast cancer earlier than the day workers. The time between baseline and diagnosis was significantly shorter among those who worked shifts with night work than those who worked days only. This might indicate that the tumor progression was faster among night shift workers. When we included only women <60 years of age, the HR increased further for those who had worked shifts with night work. This indicates that the lag between exposure and cancer is not very long and supports the hypothesis that shifts with night work is a causal factor.
Inconsistencies in previous research on night work and cancer can be due to a poor definition of the shift work variable. One null study used a definition based on occupation (42). Those who had a job title corresponding to an occupation with an estimated prevalence of ≥40% of shift workers, according to the Swedish Survey of Living Conditions, were defined as shift workers. The negative results in that study could be due to misclassification of the exposure. Given that the misclassification is non-differential, it will bias the effect estimate towards null. Another negative study asked for exposure to shift work only for the last 15 years, which probably led to misclassification of shift workers with a longer exposure time or exposure to shift work further back in time (34). Our finding that those who worked shifts with nights were diagnosed with breast cancer at a younger age, and that the time between baseline and diagnosis was shorter could have a bearing on this issue. One might hypothesize that some women have increased vulnerability due to genetic differences or behavioral factors. When they are exposed to shift work, cancer might develop early.
There are a number of risk factors for breast cancer suggested in the literature: age at birth of first child, number of children, obesity, use of contraceptive pills, hormone treatment, nulliparity, smoking, alcohol consumption, and short time of breast feeding (43, 44). We tested potential confounding by entering contraceptive pills, other hormonal treatment, menopausal status, obesity, number of children, smoking (ever and current), alcohol intake, and educational level into the analyses. Number of children and frequent alcohol intake changed the regression coefficient for shift work, and were therefore included in the final model. Yet, even if many potential confounders have been adjusted for, there may still be others not considered in the present study. Among them could be be age of first exposure to shift work. Also exposure before or after menopause could be an interesting factor, but such data were not available.
The present study has several other limitations that are common in most studies. Reliance on self-reported exposure can involve anamnestic mistakes. Our data showed that if people are asked for past experience of shift work, the information given will be affected by difficulties in correctly recalling previous exposure. Of those who reported no experience of shift work in the final follow-up, 22% actually had reported current exposure to shift work at baseline. The agreement between information given at baseline and follow-up, however, was better when considering shift work with night shifts. Of those who reported no experience of night shift work at follow-up, only 2% reported night work at baseline. It appears that retrospective information about night shift experience is more reliable than information about shifts without night work. In 53% of the subjects, we had retrospective information about lifetime exposure to shift work (with and without night work), in 36% we had only baseline information. The baseline questionnaire provided information only on current shift work/night work, and it is probable that some subjects, who were classified as day workers based on this information only, were actually former shift workers. However, it is not possible to draw any conclusions about how this misclassification could have biased our results.
Another issue is the size of the study; a larger number of participants may have yielded clearer results in some of the analyses. A strength of the present study is the longitudinal, prospective cohort design. In the literature, we found only three prospective cohort studies on the association between shift work and breast cancer. Two were positive (5, 41) and one was negative (13). The majority of published studies have been case–control studies in which possible recall bias limits the validity of exposure information. The unique person identification number available for all Swedish citizens makes it possible to obtain almost complete data from the Swedish cancer registry on cancer incidence during follow-up. Another advantage is that we had access to information on several potential confounding factors that we could take into account in the analyses.
There are a number or biological mechanisms that could explain the increased risk for cancer among shift workers but today we cannot say which mechanism is most plausible (12, 45). In order to try preventive measures, it is necessary to elucidate the mechanism behind the increased cancer risk. If the melatonin suppression is the crucial factor, one preventive method could be to reduce exposure to LAN (46). It would also be possible to use only red light at night, because red light does not interfere with melatonin production. If the major problem is the challenge of the circadian rhythm or interaction between night work and genetic predisposition, other preventive measures should be evaluated (46).
In conclusion, we found an increased risk of breast cancer among shift workers who have had night work in their schedule. Shift workers who did not have night work had a small and statistically insignificant risk compared with day workers. Our finding supports previous research reporting an increased breast cancer risk among night shift workers. However, we cannot rule out that also shift workers without night work are at a higher risk of cancer than day workers.