Diabetes is a chronic, multifactorial disease of increasing prevalence globally. The International Diabetes Federation estimated a global prevalence among adults of 8.3% in 2013 and 11.1% in 2033 (1). Although a genetic component is present in the etiology of type-2 diabetes (DM2), obesity-related aging and environmental factors – notably low physical activity and low quality diet – are also strongly at play (2).
Additional aspects of modern life have also been investigated as a possible risk factor for DM2, including night work (3). The biological mechanisms that make this a plausible association are generally associated with circadian rhythm mismatches, which may lead to metabolic problems such as increased postprandial glucose, insulin and blood pressure levels, reduced leptin action and sleep efficiency, and complete inversion of the cortisol profile. Abnormally high levels of cortisol at the end of waking and the beginning of sleeping times can contribute to hyperglycemia and insulin resistance (4). In addition, reduced leptin action stimulates appetite and lowers energy expenditure, which may lead to obesity. The combination of these effects resulting from circadian disruption, may favor increased risk of obesity, hypertension, and DM2 among shift and night workers (4, 5).
The discussion of the putative connection between night work and diabetes can benefit from an analysis of alterations in glycemic levels preceding the diagnosis of diabetes. Although relevant studies are scarce, a significant association between shift work and impaired glucose tolerance (IGT) has been observed in both longitudinal (6) and cross-sectional studies (7).
A recent meta-analysis suggests that shift work is associated with significantly increased risk of DM2, especially among men (8). According to Shiri (9), the gender differences found in this meta-analysis could be explained by confounders, selection bias, and the types of shift schedule (9).
Given the discussion of the association between work schedule and glucose metabolism (3, 10) and the need for population studies to explore gender differences (8), the aim of this study was to investigate gender-specific associations between years of exposure to night work and the presence of DM2 and IGT, using baseline data from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil).
Methods
Study population
ELSA-Brasil is a prospective cohort study designed to identify risk factors for diabetes and cardiovascular diseases. The cohort comprises 15 105 civil servants (current and retired workers), aged 35–74 years at baseline (2008–2010), who were sampled from universities or research institutions in six of Brazil’s state capitals. The Research and Ethics Committees of the institutions involved approved the study: São Paulo University, Oswaldo Cruz Foundation, Bahia Federal University, Minas Gerais Federal University, Espírito Santo Federal University and Rio Grande do Sul Federal University. All participants in the study provided written informed consent (11).
To minimize inclusion of type 1 diabetes cases in the DM2 definition, those who were diagnosed at age ≤30 years and used insulin as their first medication (N=12) were excluded (12) as were 3 participants lacking diabetes laboratory data for classification and 663 participants with missing information related to work and/or covariates.
Variables
A comprehensive set of questionnaires was applied, providing detailed information about socioeconomic conditions, habits and lifestyle, work aspects, and health. In addition, clinical measurements and laboratory tests were carried out.
A blood sample was drawn by venepuncture soon after arrival at the clinic following an overnight fast and a 75 g oral glucose tolerance test (OGTT) was then carried out with participants without known diabetes (N=14 507). Glucose was measured by the hexokinase method (ADVIA Chemistry; Siemens, Deerfield, IL, USA). Glycated hemoglobin (HbA1c) was measured using high-pressure liquid chromatography (Bio-Rad Laboratories, Hercules, CA, USA).
DM2 was classified comprehensively using blood glucose measurements and self-reported information. A participant was considered to have previously diagnosed diabetes when answering “Yes” to either “Have you been previously told by a physician that you had/have diabetes (sugar in the blood)?” or “Have you used medication for diabetes in the past 2 weeks?” Those without a previous diagnosis were evaluated for undiagnosed diabetes based on their laboratory values and then classified as having diabetes if they reached the threshold for fasting plasma glucose (FG ≥126 mg/dL) or 2-hour plasma glucose (≥200 mg/dL) or HbA1c (≥6.5%). Impaired glucose tolerance (IGT) was defined as 2-hour plasma glucose of 140–199 mg/dL (12, 13). The 14 427 participants were thus classified into three categories (i) DM2 (N=8650), (ii) IGT (N=2952) and (iii) no DM2 or IGT (the reference group, N=8650) (13–15).
Workers were classified on the basis of three questions from the questionnaire, as follows: (A) “Do you currently work, or have you worked, shifts?” – with the response options (i) “No, I have never worked shifts”, (ii) “Yes, I currently work shifts”, or (iii) “Yes, I have worked shifts, but do not work shifts anymore”; (B) “Is, or was, your shift work more often (i) day shifts only, (ii) night shifts only or (iii) mixed?” The latter option corresponds to workers who have worked days and nights over a lifetime, or those who currently work days and nights at two jobs or on rotating shifts.
Workers were classified into three groups. Exclusively day workers (reference group) were those who answered (i) to question A or (ii) or (iii) to question A and (i) to question B. The answer (i) to question “B” (day shifts only) necessarily refers to day workers, because 12-hour shifts predominate in the group. Night workers were those who answered (ii) to question A and (ii) or (iii) to question B. Former night workers were those who answered (iii) to question A and (ii) or (iii) to question B.
The following additional question was used: (C) “Which arrangement is or was most frequent: (i) 12/36-hour shift, (ii) 12/60-hour shift, (iii) weekly 24-hour shift, (iv) weekly 12-hour shift, (v) biweekly 12-hour shift or (vi) Other (please specify)?”. For the present study, workers who answered (vi) and mentioned a schedule of ≥8 consecutive hours of night work (22:00–05:00) at least four times per month were also classified as current or former night workers (according to question A). For the present study, analyses were based on the predominant schedule (question B), regardless of the specific work arrangement.
Regarding the number of years of exposure to night work, the night and former night workers answered the question “In general, how many years do you work, or have you worked, shifts?”
The questionnaire also provided information on age at baseline, sex, education (high school or university), monthly per capita income (low, medium and high tertiles), smoking status (never, former and current smoker), alcohol consumption (none, moderate and excessive consumption; the latter defined as >210 g alcohol/week for men and ≥140 g alcohol/week for women), leisure physical activity [none, moderate, high; obtained using the International Physical Activity Questionnaire, (IPAQ)], sleep quality (difficulty falling asleep, or waking up and difficulty going to sleep again during the previous 30 nights) and working hours (“In general, how many hours do you work per week – including overtime and any other job or paid activity?”). Weight, height, and waist measurement were collected using standard equipment and techniques. Body mass index (BMI) was defined as kg/m2 (14).
Statistical analysis
For the descriptive analyses, categorical variables were expressed as percentages and continuous variables as mean and standard deviation (SD). Generalized additive models (GAM) were used to determine cut-off for total number of years of exposure to night work for men and women. GAM extend generalized linear models by including nonparametric smoothing functions (16). The dependent variable displayed a binomial distribution, and a smoothing function was included for the exposure variable. Two binomial models were fitted, the first considered the categories (i) presence of DM2 and (ii) absence of both DM2 and IGT (thus excluding those with IGT). For the second model, the categories were (i) presence of IGT and (ii) absence of both DM2 and IGT (thus excluding those with DM2).
Multinomial logistic regression analysis was performed to test the association between the exposure variables (work schedule and categories of years of exposure to night work) and the multinomial outcome (normal, DM2 and IGT). Odds ratios (OR) and 95% confidence intervals (95% CI) were estimated from multiple models, beginning with an unadjusted model, and then progressively adding sociodemographic factors (model 2), behavioral and work-related characteristics (model 3), and lastly BMI and waist circumference (model 4). Following the a priori hypothesis, we tested for interactions between gender and night work and planned separate analyses by gender. All analyses were performed using the software R, version 2.15.
Results
In the baseline study sample (N=14 427) of the ELSA-Brasil cohort, 19.6% (N=2825) were classified as having DM2 and 20.5% as having IGT (N=2952). Mean age was 52.1 (SD 9.1) years. A total of 11 699 (81.0%) individuals had never worked at night, 821 (5.7%) were night workers at interview and 1907 (13.2%) were former night workers. Baseline glucose tolerance for men and women is shown in table 1. As expected, factors traditionally associated with DM2 – such as high BMI and waist circumference, physical inactivity, low education degree and income – were more frequent among those with diabetes. Longer working hours were observed among men than women, but no differences were observed in either sex in the relation between mean weekly hours worked and the outcome. As regards exposure to night work, 7.6% of the women and 6.6% of the men classified as having DM2 had worked at night for >20 years.
Table 1
Sociodemographic, behavioral and work-related characteristics by gender, DM2 (type-2 diabetes) and glucose tolerance. ELSA-Brasil (2008–2010). [BMI=body mass index; FG=fasting glucose; HbA1c=glycated hemoglobin; IGT=impaired glucose tolerance; SD=standard deviation; Waist=waist circumference]
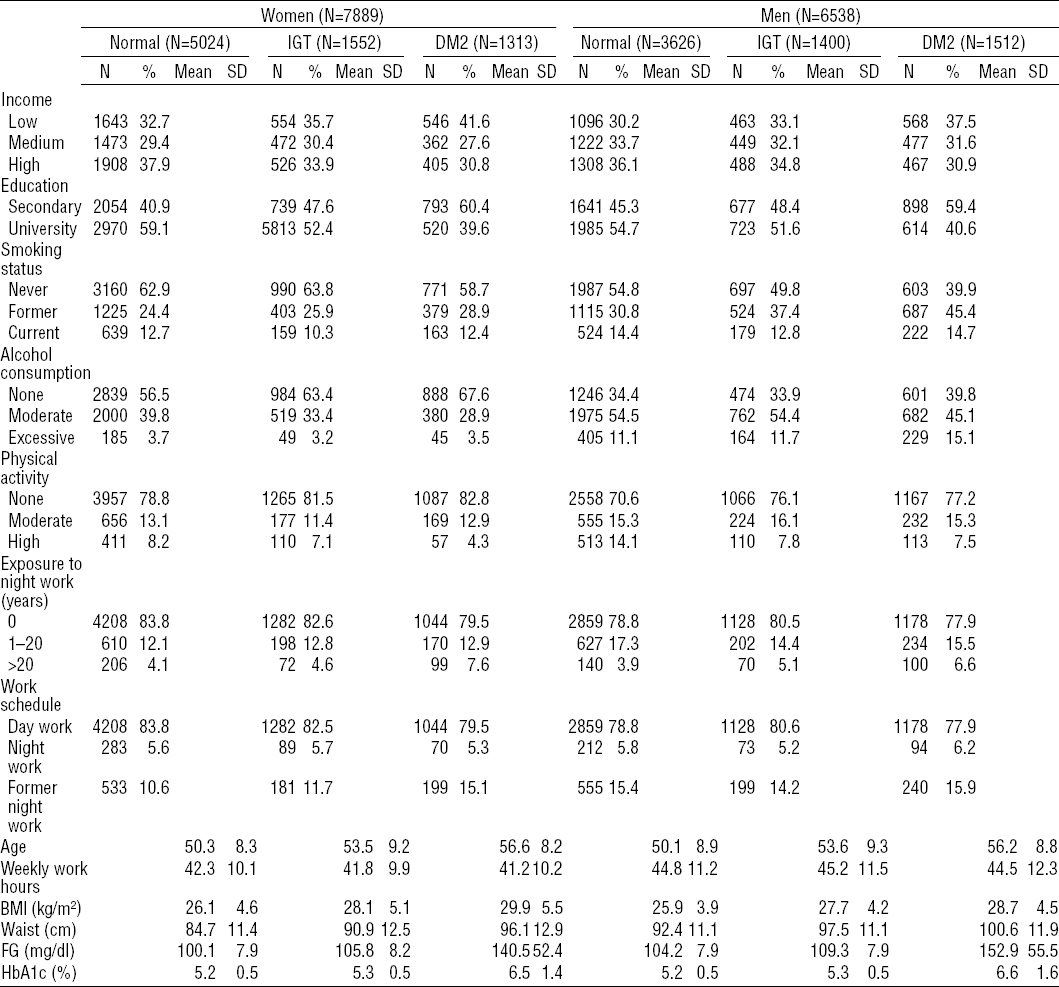
As shown in figure 1, after adjusting for all potential confounders, it was only after 20 years of exposure (solid line, change in curve pattern) that a (non-significant) trend was observed among men for a positive association between night work and the outcomes (DM2 and IGT). Among women, in contrast, there was no specific cut-off value; the association between DM2 and years of exposure to night work increased in a linear fashion.
Figure 1
Increments in type-2 diabetes and impaired glucose intolerance (IGT) by years of exposure to night work, estimated using generalized additive models. The solid black line represents the regression line and the dotted lines represent the 95% confidence interval. The bold dotted line indicates that there is no association.
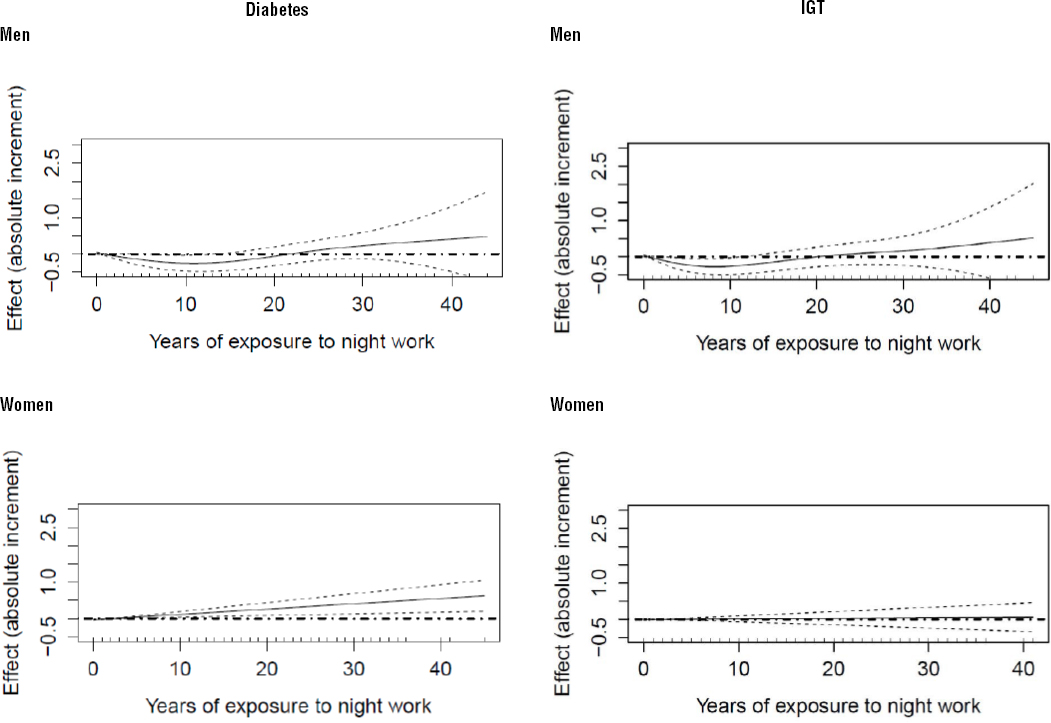
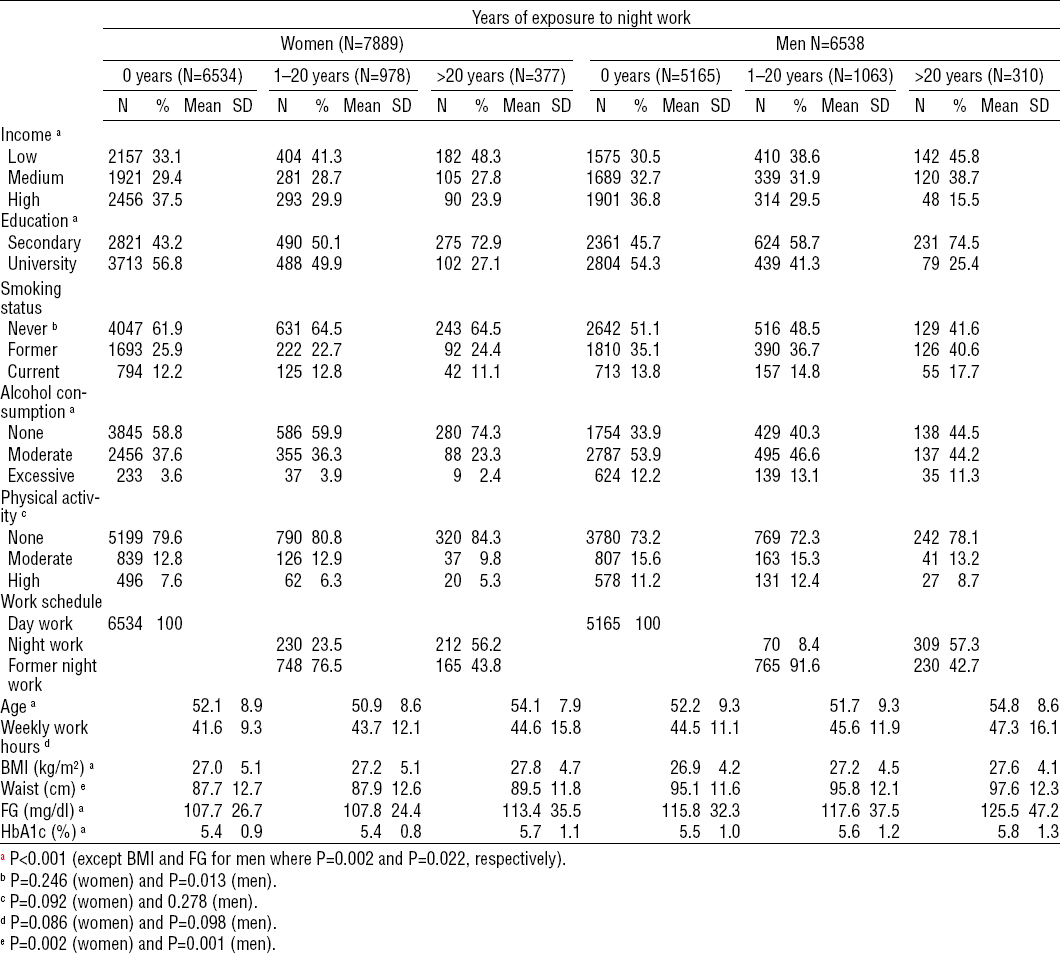
Table 2 shows the sample characteristics by category of years of night work. In all, 2041 (14.1%) participants worked at night for 1–20 years and 687 (4.8%) for >20 years. Among both women and men, exposure to night work for >20 years was associated with less education, lower income, and higher BMI, fasting glucose, glycated hemoglobin, and waist circumference.
Table 2
Sociodemographic, behavioral and work-related characteristics, and glucose tolerance by gender and years of night work. ELSA-Brasil (2008–2010). [BMI=body mass index; FG=fasting glucose; HbA1c=glycated hemoglobin; SD=standard deviation; Waist=waist circumference].
Multinomial logistic regression was used to test interaction by gender in the association between DM2/IGT and years of night work, after adjusting for age, income, education, smoking, alcohol consumption, leisure physical activity, working hours, sleep quality, BMI, and waist circumference. A statistically significant interaction (P=0.011) was observed with sex categories and, therefore, analyses were performed separately for men and women. Among women who worked at night, the odds of DM2 and IGT were 15% (OR 1.15, 95% CI 1.13–1.17) and 9% (OR 1.09, 95% CI 1.07–1.11), respectively, greater than among women who had never worked at night. Among female former night workers, the odds of DM2 and IGT were 31% (OR 1.31, 95% CI 1.18–1.44) and 5% (OR 1.05, 95% CI 0.93–1.18), respectively, greater than among women who had never worked at night. Among men, the odds of DM2 and IGT were no greater among night workers and former night workers than among men who had never worked at night. The OR values for DM2 and IGT were 0.94 (95% CI 0.92–0.96) and 0.84 (95% CI 0.83–0.86), respectively, for night workers as compared with men who had never worked at night. Male former night workers showed OR of 0.80 (95% CI 0.72–0.88) and 0.82 (95% CI 0.73–0.91) for DM2 and IGT, respectively, as compared with those who had never worked at night.
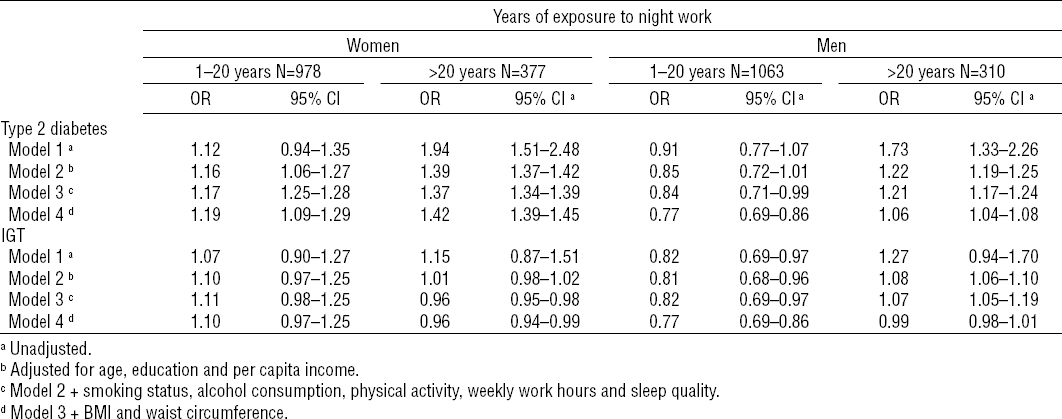
Considering the total number of years of exposure to night work (table 3), after adjusting for all potential confounders, the OR for DM2 and IGT among women exposed to night work for >20 years, as compared with those who had never worked at night, were 1.42 (95% CI 1.39–1.45) and 0.96 (95% CI 0.94–0.99), respectively. Among men, the OR for DM2 and IGT were 1.06 (95% CI 1.04–1.08) and 0.99 (95% CI 0.98–1.01), respectively, for those exposed to night work for >20 years, as compared with those who had never worked at night (table 3).
Table 3
Association between type 2 diabetes and impaired glucose tolerance (IGT) by gender years of exposure to night shift work. ELSA-Brasil (2008–2010). Derived from multinomial logistic regression models. Reference group: workers who had never worked night shifts (N=6534 and 5165 for women and men, respectively). [OR=odds ratio; 95% CI=95% confidence interval]
When considering only those who were unaware of having diabetes, after adjustment for all potential confounders, the results were similar: among women who worked at night, the OR of DM2 and IGT were 1.25 (95% CI 1.23–1.26) and 1.10 (95% CI 1.07–1.12), respectively. Among men, the OR of DM2 and IGT were 0.83 (95% CI 0.82–0.87) and 0.83 (95% CI 0.81–0.85), respectively, as compared with those who had never worked nights.
Discussion
We observed an association between night work and diabetes after adjusting for sociodemographic variables and behavioral and work-related factors. Among women, higher odds of DM2 were observed among both those exposed to night work for 1–20 years and those with >20 years’ exposure, when compared with the unexposed. Among men, higher odds of DM2 were observed only in the group exposed to night work for >20 years. These results confirm the association between night work and DM2, and suggest that it is stronger in women than men.
In this connection, after adjusting for age, education and psychosocial stress, Eriksson et al (17) also observed increased risk of diabetes among women in shift work (OR 2.20) but not men (OR 0.90). This risk was lower after additional adjustment for family history of diabetes, BMI, physical activity, smoking, and marital status [OR 1.9 (95% CI 0.8–4.4) and 0.8 (95% CI 0.4–1.7) for women and men, respectively] (17). In this present study, a protective factor tendency was also observed for men. The scarcity of gender-stratified studies makes it difficult to compare results. Eriksson’s results and ours are at odds with a recent meta-analysis of the association between shift work and diabetes, which showed significantly higher increased risk of diabetes among men (8). On the other hand, Shiri (9) argued that the gender differences found in this meta-analysis may be explained by selection bias and/or differences in exposure variable (rotating or fixed night work) for men and women (9). The author also refers to the confounding variables in the studies, since all the studies with women controlled for BMI and physical activity, whereas half the studies did not control for these two factors among men (9). In the present study, adjusting for BMI and waist circumference reduced the OR in the male sample, thus confirming that non-inclusion of BMI as a confounder may have contributed to over-estimation of the risk among men. This discussion warrants further studies of how the biological mechanisms of shift work and diabetes are affected by gender as mentioned previously (8).
Gender differences were reinforced by data on former night workers: prior exposure to night work was associated with higher odds of the outcome of interest occurring among women but not men. These results underline that the cumulative adverse health effects of night work – in this case in relation to DM2 – are more marked among women. In addition, as shown in figure 1, the effect of exposure to night work appears to take longer to manifest itself among men, suggesting that the relationship between night work and glucose metabolism is different in men and women. As previous studies generally examine only one of the sexes or both together, the increased risk observed among women in some of the studies was possibly diluted by the risk for men (17).
In an all-male sample, Ika et al (18) found an association between rotating night shift work and diabetes, stressing that the effect of the association was more marked among the older workers, that is, among those with greater exposure to shift work. Among Japanese workers, rotating night shift work was also associated with raised glycated hemoglobin levels, revealing itself to be an independent risk factor for impaired glucose metabolism (19). Suwazono et al (20) observed the effect of rotating shift work (including the night shift) on developing diabetes in an exclusively male cohort. Similarly, in a cohort of male Japanese workers, higher incidence of diabetes was observed among those working rotating shifts (morning, then evening) as compared with those on fixed day work. In the same cohort, however, no such association was detected among workers on three-way (morning, evening and night) rotating shifts (21). These results reveal that some inconsistencies in the literature are due to the difficulty in comparing studies since different types of work schedules result in different risks on health (6).
Meanwhile, in a retrospective cohort of Italian workers, Biggi et al (22) found a negative association between fixed night work and glucose levels (coefficient = −4.2, P<0.05). This effect of night work on glucose levels is similar to the results of this present study in which the odds of DM2 and IGT for men were lower among former night workers, current night workers, and those exposed to night work for 1–20 years, as compared with workers who had never worked at night. It’s possible that those most susceptible to diabetes died earlier from related disorders such as cardiovascular disease, and that this occurred more among men. These results, suggesting that night work is a protective factor against diabetes among men, clearly deserve further longitudinal investigation. It should be noted that for men exposed to night work for >20 years, DM2 was slightly higher (6%) compared to workers who had never worked at night; the exposure to night work for >25 years increased the odds of DM2 by 16% (data not shown), once again highlighting inconclusive results.
As regards women, the odds of IGT in the present study were higher among both former and current night workers and those who had worked nights for 1–20 years, as compared with those with no experience of night work. The association was slightly lower among those who had worked night shifts for >20 years, perhaps because, with time, those at higher risk had already developed diabetes or died of related disorders such as cardiovascular disease. Alternatively, the association may represent those more resistant to glucose-related metabolic changes. In relation to DM2, significantly higher ORswere observed among women with longer exposure to night work: 19% and 42% for 1–20 and >20 years exposure, respectively. In a cohort of nurses with 18–20 year follow-up, Pan et al (23) found increasing risk of DM2 with years spent working rotating night shifts. Compared with women who had never worked alternating night shifts, the risk of DM2 among participants with 1–2, 3–9, 10–19 and ≥20 years’ exposure in that working arrangement, after adjusting for age, was 1.04, 1.24, 1.55 and 1.78, respectively. Adjusting for age, alcohol consumption, physical activity, smoking, race, menopause, contraceptive use, history of diabetes and diet showed that 5 years’ exposure to working alternating night shifts increased the risk of DM2 by 13%. When additionally adjusted for BMI, the risk of DM2 fell to 5% (23). Accordingly, the results were considered evidence that a prolonged period of working alternating night shifts is associated with higher risk of DM2 and that the relationship is not completely explained by BMI, which has been considered to function as a mediator (24).
Our results were adjusted for BMI and waist circumference, given that scientific evidence suggests that abdominal obesity is associated with insulin resistance and DM2 (25, 26). Furthermore, it has been shown that waist circumference coupled with BMI predicts health risk better than does BMI alone (27). Thus, these variables may function as mediators. If this is the case, the interpretation of the magnitude of the associations found in the final model (model 4) is conservative, since it assumes that BMI and waist circumference are confounders. We found that the adjustment yielded greater effect among men than among women: OR for DM2 changed from 1.21 (model 3) to 1.06 (model 4) among men and from 1.37 (model 3) to 1.42 (model 4) among women. Therefore, BMI and waist circumference seem to have greater influence among men.
The role of potential confounders in previous studies of shift work and diabetes deserves further attention. The influence of confounders is discussed in a review article, which points out that the findings on the association are inconclusive, partly because of the lack of uniformity in the adjustments applied in the studies (3). In a systematic review article on shift work and metabolic syndrome, Canuto et al (28) discuss the fact that associations are found in the crude analyses. When potential confounders are taken into consideration, however, the evidence is inconclusive, pointing to a need for studies of the role of lifestyle variables as mediators or potential confounders of the association (28).
A recent meta-analysis on the association of shift work with diabetes found that the studies were heterogeneous in terms of the adjustments for confounder variables, but the subgroup analyses showed that the associations are more evident when not adjusted for BMI and physical activity (8). As argued by Canuto et al (24) these variables may be intermediate in relation to the effect of shift work on metabolic diseases, since they are strongly influenced by shift work. Therefore, the variables related to lifestyle may be considered as mediators in the association between shift work and DM2.
Puttonen et al’s model (29) gives grounds for the claim that the continuous circadian stress resulting from exposure to shift and night work may cause excessive secretion of cortisol, catecholamines, and interleukins, which together with increased insulin concentrations, is considered to lead to abdominal fat build-up, insulin resistance and lipid disorders. Exposure to night work, together with cessation or decrease in melatonin secretion, also alters the temporal organization of metabolic functions, because melatonin release follows a daily pattern which is important in maintaining the circadian synchronization between the activity/feeding and repose/fasting rhythms (30). Although glucose and insulin levels are related to feeding, there is a circadian variation (in which daily rhythms of insulin and blood glucose secretion feature higher levels in the early morning), which also indicates the participation of endogenous factors, such as melatonin, in regulating these levels. Circadian mismatch resulting from night work comprises higher cortisol levels and lower melatonin levels at night and, thus, result in reduced secretion of leptin, hyperglycemia, and insulin resistance (4).
Regarding gender differences, studies of diabetes (31), metabolic syndrome (32) and cardiovascular disease (33) point to possible different impacts by sex, while Wong et al (34, 35) and Puttonen et al (36) have discussed aspects relating to work schedule. In a study of shift work and metabolic syndrome, Puttonen et al (36) pointed out that, although the reasons for the observed gender differences are not completely known, the characteristics of the working conditions of men and women may influence the associations found. Gender differences across some industries, such as healthcare and manufacturing, may also result in different hazard exposures among men and women and influence the risk of work injury (35). Concerning the sociodemographic and behavioral aspects, gender differences were examined in this present study, but no results were observed to explain the differential odds of DM2 among men and women. Our prior findings (data not yet published) indicate that working at night is related to metabolic alterations that are connected with the development of DM2 and differ between men and women. Although higher levels of fasting glycaemia were observed among men, the proportion with impaired fasting glycaemia (>100 mg/dl) was higher among night than day workers only among the women. Accordingly, with regards DM2, the findings on fasting glycaemia point to a higher probability of DM2 among women.
It is possible, however, as Choi et al (37) argued, that some unexamined genetic or environmental risk factor may play an important role in explaining these findings, which would represent one limitation of this present study. Gender differences related to sleep complaints may support the observed results. The relation between night work and DM2 may be mediated through short sleep duration. Suarez et al (38) demonstrated that indexes of sleep disturbance are associated with greater psychological distress, higher levels of fasting insulin, fibrinogen, and inflammatory biomarkers, but only among women. Also, differences in responses of men and women to shift work based on circadian rhythms of melatonin and cortisol should be examined. Unfortunately, details of sleep patterns were not measured at wave 1 of ELSA-Brasil.
Another important possible limitation of this study is reporting bias as participants were asked to recall their history of shift work. Night workers who had left such a work schedule many years previously may have reported their experience differently from current night workers. Also, the information obtained with regard to work schedules does not distinguish workers on alternating night shifts from those on fixed night shift, and possible errors in classifying exposure might be present. Biggi et al (22) suggested that caution should be used when comparing findings for fixed night workers and alternating shift workers because permanent night workers may adopt more stable living habits than alternating shift workers with regards to sleep and meal times. However, circadian stress on days off work when the routine reverts to standard times, should also be considered (22).
Finally, this study has some strengths. First, the laboratory assessment was very comprehensive, which made it possible to identify fully new cases of DM2 and those with intermediate hyperglycemia. The analysis allowed diabetes to be distinguished from intermediate hyperglycemia, thus adding relevant information since the inclusion of cases of IGT in the reference group may underestimate the risks; (2) gender-based analysis enabled different patterns of association to be observed in the two groups; (3) adjustment for BMI and waist circumference allowed the possible mediator effect of those variables to be analyzed; and (4) analyses by years of exposure to night work had an advantage over only using the variable “work schedule”, because it permitted more detailed evaluation of the data for men, which revealed an association only after 20 years’ exposure. Finally, analyses including only those who were unaware of having diabetes minimized the possibility that the associations found were due to changes resulting from a diagnosis of diabetes.
Overall, the findings contribute to the discussion of two important issues in the field: the relations between night work and DM2 by gender and refinement of the necessary adjustments in order to test for such associations. Longitudinal studies drawing on the ELSA-Brasil study will be able to corroborate or refute the associations identified here.
Funding
The Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science and Technology (Financiadora de Estudos e Projetos and CNPq National Research Council) supported the ELSA-Brasil baseline [grants 01 06 0010.00 RS, 0106 0212.00 BA, 01 06 0300.00 ES, 01 06 0278.00 MG, 01 06 0115.00 SP, 01 06 0071.00 RJ]. The first author is the recipient of scholarships from Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ - E26/100.448/2014). The funding source had no influence on study design, data collection, analysis and interpretation, writing the paper nor in the decision to publish, and the authors declare no conflict of interest.



