Shift work may be defined as the organization of working time to cover more than the usual 8-hour workday, up to a 24-hour period (1). Some epidemiological studies have used three night shifts per month to classify exposure to night shift work (1) although no standard definition exists. Shift work is prevalent in healthcare, emergency services, manufacturing, retail, and hospitality. Some jobs require regular work on the same night shift (ie, permanent night shift), while others are employed on rotating shift schedules involving days and nights. Approximately 15–20% of the working population in Europe and North America is employed in either a permanent night or rotating shift schedule (2).
Shift work, particularly work at night, has been found to disrupt endogenous circadian rhythms involved in melatonin expression, sleep patterns, food digestion, and other physiological processes (2). Work at night is associated with a range of known and potential adverse health effects. In 2007, the International Agency for Research on Cancer (IARC) classified shift work involving circadian disruption as a probable human carcinogen (group 2A) based on sufficient animal evidence and limited human evidence (2). The epidemiological studies considered in IARC’s evaluation showed increased risks of breast cancer among long-term rotating shift workers and emerging evidence for other cancer types, such as prostate and colorectal (2). Since the IARC decision, several meta-analyses have been published, one supporting the association between shift work and breast cancer (3) and two reporting inconclusive evidence (4, 5). Aside from potential cancer risks, shift workers also experience increased incidence of chronic illnesses including cardiovascular disease, diabetes, and metabolic syndrome (a combination of obesity, dyslipidemia, high cholesterol, and insulin resistance) (6), as well as gastrointestinal disorders (7), workplace injuries (8), and disruption of family and social life (9).
The short- and long-term effects of shift work on sleep have also been studied. Night work has been shown to reduce sleep quantity and quality on workdays and days off. While shift workers tend to fall asleep rapidly in the morning immediately following a night shift, sleep tends to be shorter due to the natural awakening effects of circadian rhythms during the daytime, as well as social cues and daytime commitments. Objective assessments using electroencephalography (EEG) readings show a decrease in rapid eye movement (REM) sleep and stage-two sleep (10). Sleep questionnaires completed by shift workers show reduced sleep length and higher frequencies of sleep difficulties, intermittent sleep, and early waking (11). Poor sleep quality and quantity have been shown to be related to various chronic diseases (12) including diabetes (13), cardiovascular disease (14), and obesity (15, 16). Thus, sleep quantity and quality are important outcomes of interventions aimed at improving long-term health among shift workers.
There is a need for interventions that can be implemented in workplaces – or by workers outside of work hours – to mitigate the harmful effects of shift work. Laboratory and field-based studies have been conducted to evaluate preventive approaches and interventions that promote health. To date, studies have assessed: (i) shift schedule changes (eg, direction of rotation, speed of rotation, shift length, and self-rostering); (ii) controlled exposure to light and dark (eg, exposure to bright light in the workplace, use of goggles to minimize bright light exposure after night shift work and before sleep); (iii) behavioral or lifestyle interventions (eg, dietary changes, physical activity, scheduled napping); and, (iv) pharmacological aids or other substances to facilitate sleep (eg, exogenous melatonin) or to enhance alertness (eg, Modafinil, caffeine).
Reviews have summarized the effects of specific intervention types such as caffeine (17), bright light and melatonin (18), and changes in shift schedules (19), however these reviews included laboratory-based studies that were conducted among non-shift workers in simulated night shift environments, and findings may not be generalizable. They also included studies that examined outcomes likely irrelevant to long-term health, such as productivity and absenteeism. To our knowledge, there is currently no comprehensive review focused exclusively on data collected from prospective interventions conducted among shift workers with the aim of improving long-term health. A summary of this evidence would help to identify potentially effective interventions and gaps for further research.
The primary objective of this review was to synthesize the research reporting interventions that have been implemented among shift workers designed to prevent the long-term, adverse health effects of shift work. A secondary aim was to evaluate the overall quality of included studies. Based on the findings, future directions for intervention research are suggested.
Methods
Search strategy
A comprehensive list of MeSH terms related to shift workers, health-based interventions, and long-term health outcomes were developed (Appendix, www.sjweh.fi/data_repository.php) and used to search MEDLINE, CINAHL, and EMBASE for studies published on or before 13 August 2012. The search was limited to studies that were conducted on human subjects and published as English-language articles in peer-reviewed journals. Reference lists of relevant review papers and studies identified in the literature search were hand searched for other potentially eligible articles.
Study eligibility and selection
Two reviewers independently inspected the title and abstract of each study identified to determine eligibility for inclusion. Eligibility was based on a pre-determined set of criteria (Appendix, www.sjweh.fi/data_repository.php). Studies were included if the intervention aimed to improve one or more chronic disease-related health outcomes among shift workers. Participants must have been working permanent or rotating night shifts at the time of intervention. Interventions that were implemented in simulated work environments or non-shift workers (eg, healthy volunteers) were excluded. Interventions that were conducted among workers with extreme work schedules (eg, >24 hours of continuous work) or workers who cross time zones (eg, astronauts, aircrew, military workers) were excluded because of potential confounding from factors such as cosmic radiation and jet lag.
The intervention must have been implemented for ≥7 consecutive days since this review focused on interventions with implications on long-term health. Before-and-after studies, or natural interventions (defined as studies involving an intervention not initiated by researchers) were included if there was at least one main outcome measured both pre- and post-intervention in order to determine the effect of the intervention itself. We included non-pharmacological and pharmacological interventions. Randomized and non-randomized study designs were included, as well as case–control, and cohort studies if the exposure was an intervention.
Eligible studies were required to report on outcomes related to chronic disease risk as defined by the World Health Organization (WHO): “diseases of long duration and generally slow progression” (20). The related health outcomes included were: (i) sleep quantity and quality; (ii) markers of circadian disruption/adaptation; (iii) biological markers of chronic disease; and (iv) common modifiable risk factors for chronic disease as identified by the WHO (20) (Appendix, www.sjweh.fi/data_repository.php). Studies only reporting organizational outcomes (eg, productivity, absenteeism) were excluded because they were beyond the scope of this review’s focus on shift workers’ health. Similarly, studies that only measured work-related injuries were excluded because this outcome has a different etiology than chronic disease. Although the experience of sleepiness and fatigue are part of the diagnosis of shift work sleep disorder (21), these outcomes were excluded in this review since they are more strongly related to work-related injuries and productivity than chronic disease risk, which is linked with the measures of sleep quality and quantity that are included here. Mental health and psychosocial outcomes such as psychological stress, work-life balance, burnout, mood, and well-being were also excluded. Although these are interesting and important outcomes, they represent a distinct set of health effects that have different risk factors and etiologies compared to chronic disease as defined in this review. Outcomes such as “attitudes towards intervention” were omitted since these were primarily concerned with the intervention itself and not shift workers’ health.
The two reviewers each generated a list of eligible studies that were compared, and eligibility of any paper in question was resolved by consensus. Included papers were obtained in full and further reviewed for data extraction and quality assessment.
Quality assessment
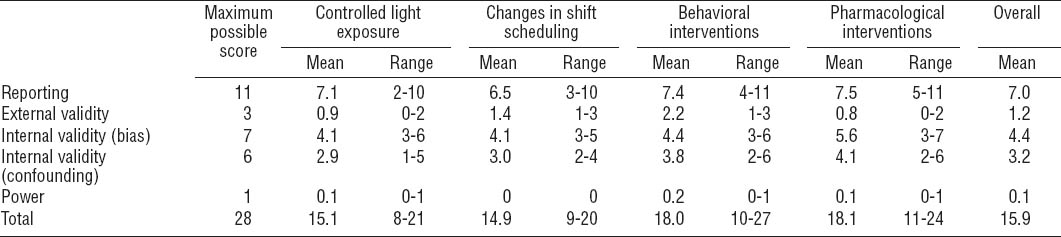
Study quality was assessed using a 28-point checklist adapted from Downs & Black, with reported test-retest and inter-rater reliability of 0.88 and 0.75, respectively (22). The original checklist has been widely used in systematic reviews of both randomized and non-randomized studies. Of the various quality assessment tools available, this was the most appropriate tool as it has been validated, and it was not possible to randomize workers in many of the included studies. The checklist encompasses four key areas with the following number of points available: (i) reporting of objectives, outcomes, study subjects, interventions, confounders, results, adverse events, loss to follow-up, and probability values (11 points); (ii) external validity (3 points); (iii) internal validity: a) bias in the measurement of the intervention and the outcome (7 points), and b) confounding related to the selection of study subjects (6 points); and (iv) statistical power (1 point). Two reviewers independently completed the checklist and gave each study a score for each section, and an overall score. Scores assigned by each reviewer were compared and discrepancies were resolved by consensus. Aggregate scores for intervention types are presented for different intervention types or sub-types in order to identify areas for improvement in subsequent research. Individual quality scores are published in the Appendix (http://www.sjweh.fi/data_repository.php).
Data extraction and synthesis
Included studies were grouped as one of four intervention types: controlled light exposure, shift schedule, behavioral, and pharmacological. Detailed information about the objective, design, sample, intervention, comparison group, and outcomes were extracted from each publication and tabulated independently. Only health outcomes that met eligibility criteria were extracted. Adverse events and funding sources were noted. It was not possible to conduct a meta-analysis due to the heterogeneity of study designs, populations, interventions, and outcomes. Authors were not contacted for additional information about their studies. Missing information was noted.
Results
The literature search generated 5053 search results. Of these, 4425 titles and abstracts did not meet inclusion criteria and were excluded (Appendix, figure 1, www.sjweh.fi/data_repository.php). Fulltext articles were obtained for the remaining 628 search results. Of these, 584 were excluded. The most common reason for exclusion was laboratory or simulated interventions conducted among non-shift working volunteers. Hence, this review included 44 articles describing results from 38 different interventions published between 1982 and 2012.
Demographic characteristics
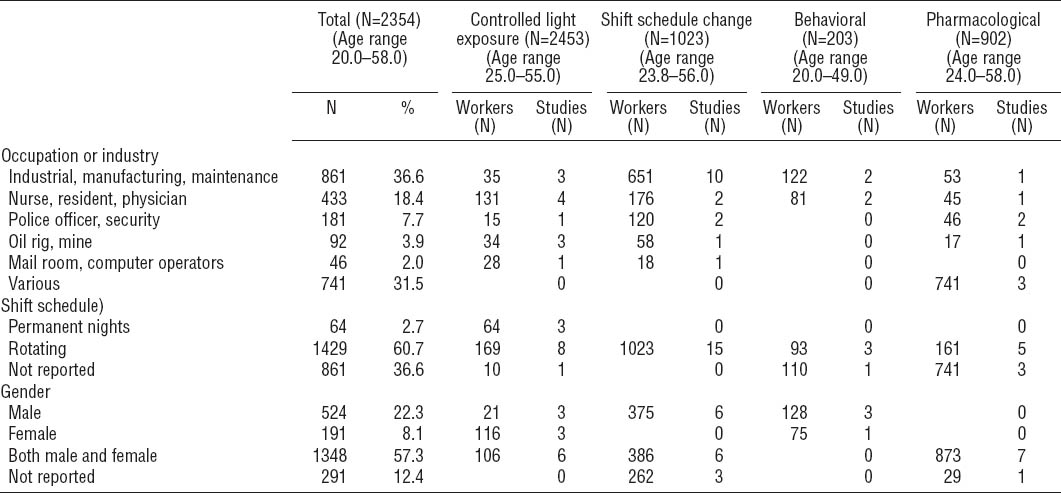
Studies included a total of 2354 workers (table 1). One-third (36.6%) were industrial or manufacturing workers, followed by healthcare workers (18.4%), police officers and security workers (7.7%), and workers in other occupations and industries (37.4%). Most worked rotating shifts (60.7%); only 2.7% worked permanent night shifts (remainder, not reported). Studies that assessed changes in shift schedules recruited the largest number of workers (N=1023) compared to studies of controlled light exposure (N=243), behavioral interventions (N=203), and pharmacological interventions (N=902). Reports included more men (54.0%) than women (30.4%). Shift workers’ age ranged from 20–58 years.
Table 1
Characteristics of participants in included studies. Percentages across study types may exceed 100% due to one study (24) that included both controlled light exposure and pharmacological interventions
Quality assessment
The average rating across all studies was 15.9 out of a possible 28 points (range: 8–27) (table 2, for individual scores see Appendix, tables A–D, http://www.sjweh.fi/data_repository.php). For reporting, scores ranged from 2–11 (mean 7.0) out of a possible 11. Information was most frequently missing for the distribution of principal confounding factors in study groups, adverse events, and p-values for statistical tests. External validity scores ranged from 0–3 (mean 1.2) out of a possible 3, with reviewers frequently unable to determine whether participants were representative of shift workers as a whole or of workers in specific industries under investigation. Internal validity (bias) scores ranged from 3–7 (mean 4.4) out of a possible 7. Particular concerns were insufficient information about compliance and lack of blinding of subjects and assessors. Scores for internal validity (confounding) ranged from 1–6 (mean 3.2) out of a possible 6. Deficiencies were most common regarding randomization, concealment of group allocation until complete baseline assessment, and reporting loss to follow-up. Only three interventions reported a sample size calculation.
Controlled light exposure
The literature search yielded 16 papers that described 12 interventions of controlled light exposure among shift workers (23–38) (table 3). Mean study quality was 15.1 (range: 9–21). The use of intermittent bright light was evaluated in 7 studies (23, 24, 29, 30, 32, 33, 36, 38), 4 used a combination of bright light and light-blocking goggles (25–28, 31, 35, 37), and another evaluated glasses that filtered blue light wavelengths (34). Across all interventions, light intensity ranged from 200–10,000 lux, and cumulative exposure times per shift ranged from 10 minutes to 6 hours. Follow-up ranged from 7–96 days (mean=23.7 days, median=14.0 days). The most common outcomes were sleep (N=9) (23–25, 29, 33–37) and markers of circadian rhythm: melatonin (N=7) (26–29, 32, 33, 35, 37, 38), cortisol (N=2) (31, 32, 38), and body temperature (N=3) (27, 28, 30, 32, 38).
Table 3
Controlled light exposure interventions. [+ = positive change; - = detrimental change; x = no change; ref=reference/comparison group; BL=bright light; BMI=body mass index; F=female; M=male; PSG=polysomnography; SW=shift work; VAS=visual analogue scale]
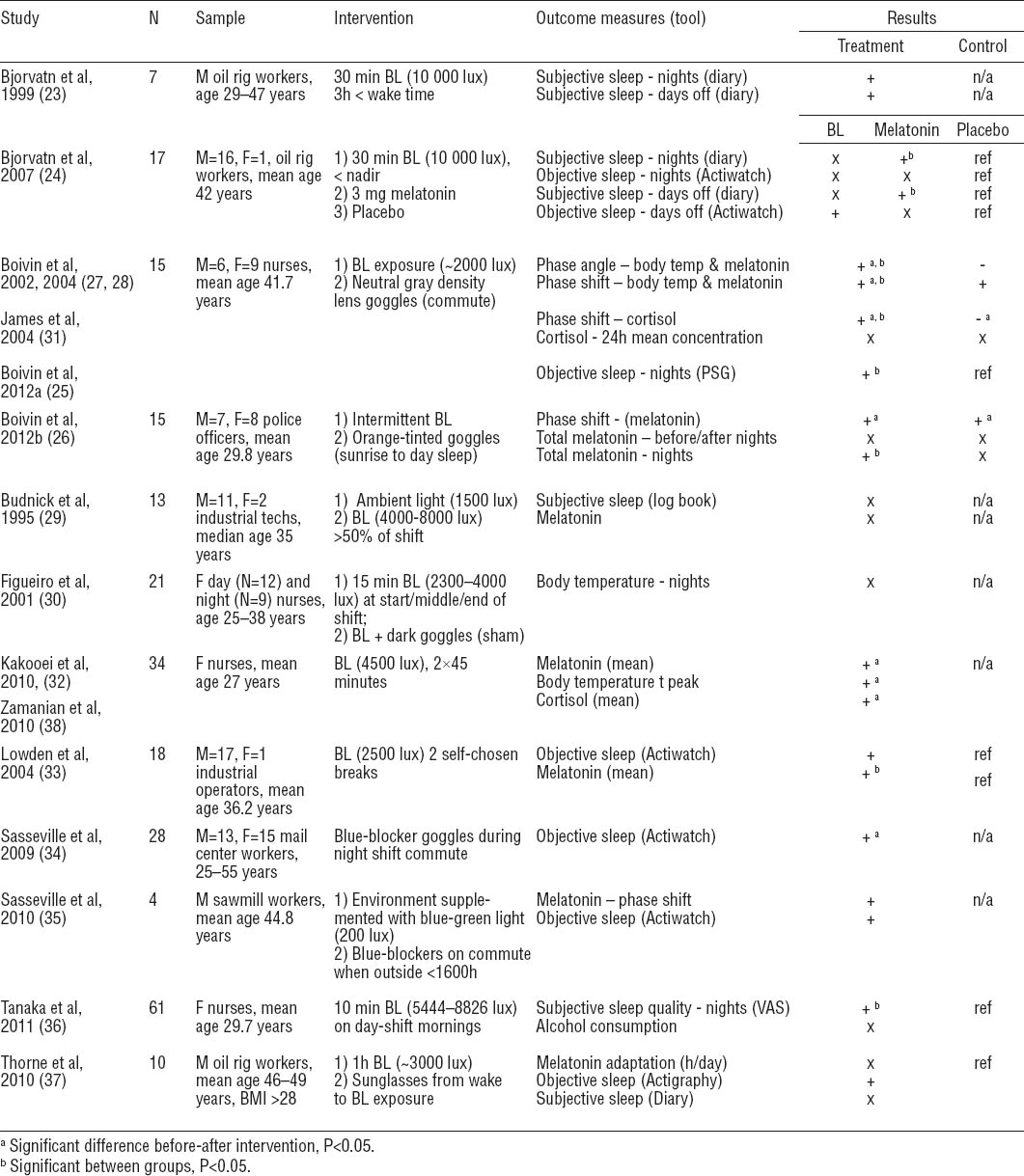
Controlled light exposure had different effects on health. Two brief periods of bright light significantly affected 24-hour total sleep time (including naps) among truck plant workers, but did not change sleep efficiency or quality (33). Among oil workers, bright light at the rig and on days off improved sleep latency and total sleep time (23), and oil workers who also wore sunglasses improved sleep efficiency (37). Nurses who were exposed to bright light before the midpoint of peak melatonin concentration and who wore goggles during the commute home increased total sleep time after night shifts (25). There was also some indication that phase shift, an indicator of circadian adaptation to night shift work, had occurred, as evidenced by significant body temperature and melatonin changes. Nurses who exposed themselves to bright light for ten minutes on workday mornings reported significant improvements in quality of night sleep on day shifts compared to non-bright light exposure periods (36). Wearing blue-blocking goggles while commuting improved total sleep time (34) and sleep efficiency (35) in two studies. The two remaining studies found no significant effect of bright light on sleep parameters (24, 29).
Of the studies that used a bright light intervention (with or without goggles), four successfully altered melatonin levels (26–28, 32, 33, 38) and three did not (29, 35, 37), with no difference in quality scores between the two groups of studies (means 14.2 and 14.3, respectively). Cortisol was measured as an indicator of circadian rhythms in two studies; one was successful in shifting the usual release pattern of salivary cortisol (31), and the second increased plasma cortisol levels over the night shift (32, 38). Body temperature also follows the circadian rhythm and was used to assess circadian adaptation to night shift work in three studies; two effectively altered body temperature (27, 28, 32, 38), while one found no change (30). Other health outcomes evaluated are summarized in table 2.
Shift schedule change
Fifteen interventions evaluated a change in shift schedule (39–54) (table 4). Mean study quality was 14.9 (range: 9–20). Interventions involved changes from a backward (counter-clockwise) to forward (clockwise) rotating shift (N=6) (40–42, 45, 48, 49, 53) and vice versa (N=1) (44), switching from 8- to 10- or 12-hour shifts (N=6) (43, 46, 47, 50, 51, 54), adjusting the shift schedule based on ergonomic principles (39), flexible shift scheduling (53), and delaying shift start time (52). Many changes from backward to forward rotating shifts also increased rotation speed (N=4) (40, 42, 45, 53). Follow-up ranged from four weeks to one year (mean 8.3 months, median 9 months). The three most frequently evaluated outcomes were sleep (N=15) (39–54), behaviors related to chronic disease risk (eg, diet, physical activity levels, alcohol intake) (N=7) (39, 40, 45, 48–51, 53), and chronic disease risk factors (eg, cholesterol, triglycerides, blood pressure) (39, 48–50, 53).
Table 4
Change in shift schedule interventions. [+ = positive change; - = detrimental change; x = no change; ref=reference/comparison group; M=male; F=female; A=afternoon shift; BL=Bright Light; Bwd=backward; CRP=C-reactive protein; D=day shift; E=evening shift; Fwd=forward; Gl=glucose; HbA1C=hemoglobin A1C; KSQ=Karolinska Sleep Questionnaire; LSHCI=Lund Subjective Health Complaints Inventory; M=morning shift; N=night shift; o=on call; S&Y=Shiftwork and You questionnaire; SW=shiftwork; SSI=standard shiftwork index; T=training/support; TG=triglycerides; VAS=visual analog scale; W=work.
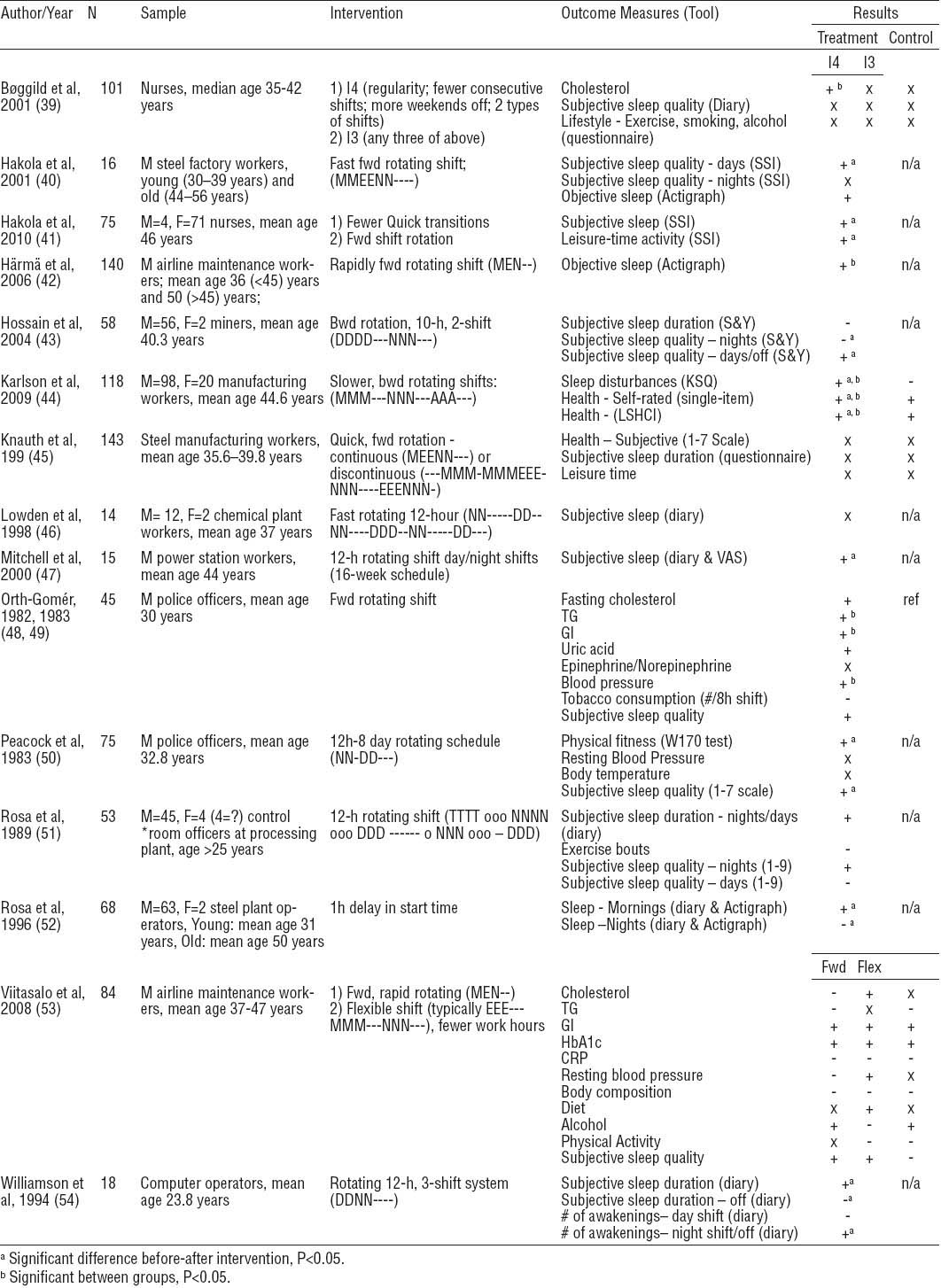
Three studies found that sleep quantity or quality was significantly positively affected by changing from backward to forward rotation (40–42), but this effect was not observed in three other reports, which found no significant effects on sleep (45, 48, 49, 53). In one study, this change was associated with significant decreases in triglycerides, glucose, and systolic blood pressure (48, 49). However, overall study quality was worse in studies that found a significant effect on sleep (mean scores 14.3 and 17.0, respectively). Of interventions that changed from 8- to 10- or 12-hour shifts, three improved sleep (47, 50, 54), one significantly improved physical fitness (50), and three resulted in no significant or negative changes in sleep after the night shift (43, 46, 51). Those which found no change were of higher quality (mean score 14.0) than those who found a significant effect (13.3). Another intervention took a multi-faceted approach to shift scheduling based on four ergonomic principles: regularity, fewer consecutive night shifts, more weekends off, and two different types of shifts. This resulted in a significant decline in low-density lipoprotein (LDL) and total:high-density lipoprotein (HDL) ratio; however, sleep quality was unaffected (39). Airline maintenance workers given individual flexibility and control over work hours experienced no significant improvement of any health parameters (53). A one-hour delay in start time at a steel plant resulted in increased sleep on morning shift days, but decreased sleep on evening and night shift days (52).
Behavioral interventions
Four interventions were implemented to modify behavior (table 5): a 1-hour rest period for electric power plant workers on the night shift (55), a physical activity program for nurses and nursing aides (56, 57), a weight loss program among aluminum plant workers (58, 59), and an educational program about strategies to enhance adaptation to shift work for emergency department attending physicians (60). The number of workers in these studies ranged from 6–110 (mean 50.8, median 43.5). Follow-up ranged from 3 weeks to 1 year (mean 21.3 weeks, median 15 weeks). Sleep was reported in three of four studies (55, 57, 60). Mean study quality was 18.0 (range: 10–27).
Table 5
Behavioral interventions. [Note: + = positive change; - = detrimental change; x=no change, M=male; F=female; FFQ=food frequency questionnaire; PSG=polysomnography; SW=shiftwork; VO2Max=maximal oxygen consumption]
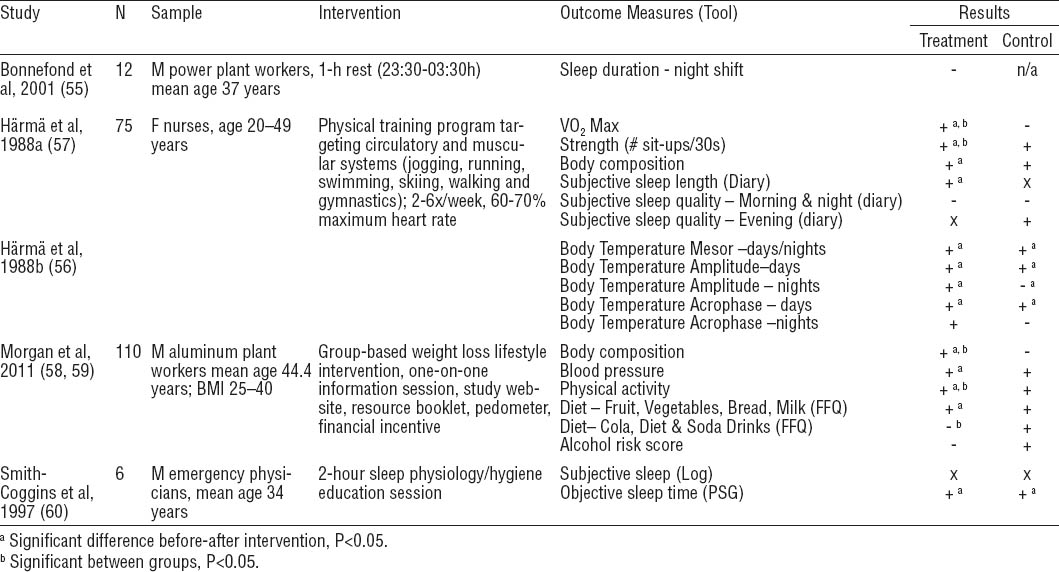
Physical activity improved sleep length with variable results on subjective sleep quality (57), and education about sleep hygiene strategies resulted in significantly improved REM sleep time (60). A 1-hour rest period during the night resulted in no significant change in sleep duration following the night shift (55). Other outcomes were also evaluated (table 5). Exercise significantly increased maximal aerobic capacity and strength, although circadian phase did not differ between groups, as measured by body temperature (56, 57). A group-based lifestyle intervention for weight loss was associated with significantly decreased body mass index and blood pressure and significantly improved physical activity and fruit intake (58).
Pharmacological interventions
Eight pharmacological interventions met inclusion criteria (24, 61–67) (table 6). Mean study quality score was 18.1 (range: 11–24). Two pharmacological agents were found to aid sleep following the night shift: melatonin and Zopiclone. Dosages of 3.0 mg (24, 62) or 5.0 mg (66) of exogenous melatonin were administered to workers in three studies. This resulted in significant sleep improvements after 14 (24) and 28 days (66) in two of three studies. Zopiclone (7.5 mg) was administered in two different study groups which reported insomnia: workers at a security company and a car manufacturing plant. Zopiclone had positive effects on total sleep time (61, 67) and quality (67), as well as sleep efficacy (61) and induction (67).
Table 6
Pharmacological interventions. [Note: + = positive change; - = detrimental change; x=no change; M=male; F=female; BL=bright light; PSG=polysomnography; SW=shiftwork; VAS=visual analogue scale]
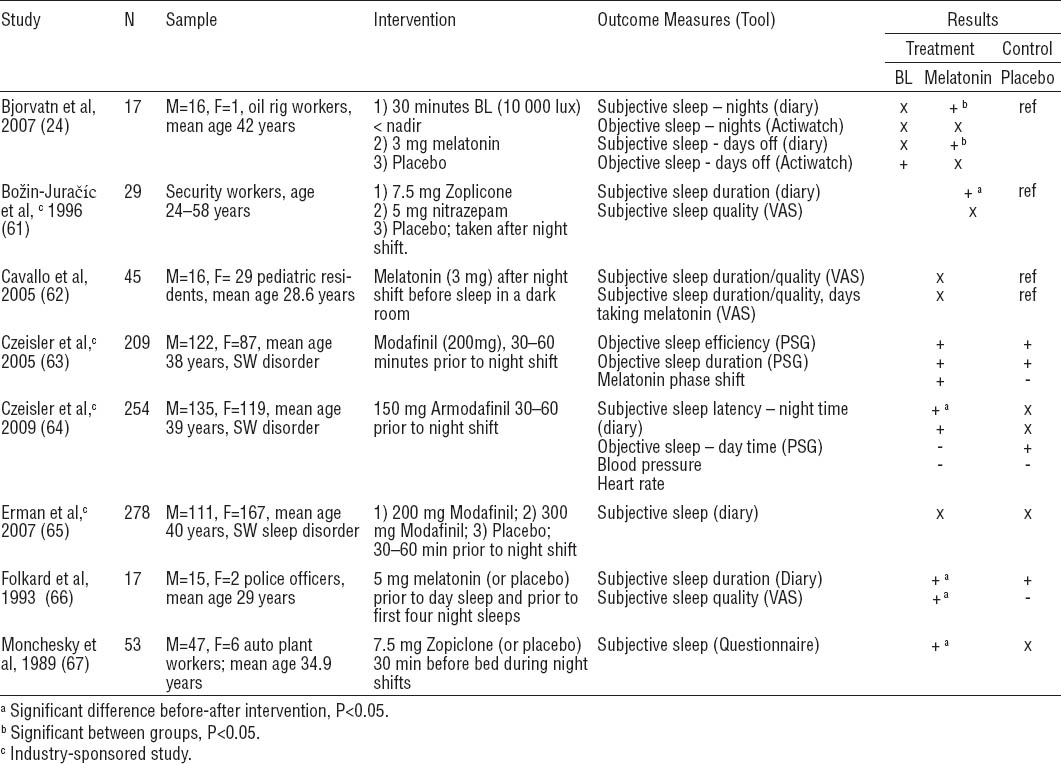
Three studies evaluated the use of Modafinil or Armodafinil as stimulants before night shifts among workers who met the defined criteria for shift work sleep disorder (63–65). Administration of 200 mg and 300 mg of Modafinil did not significantly change endogenous melatonin levels or sleep quantity before or after night shifts (63, 65). Armodafinil (150 mg) resulted in a small but statistically significant improvement in nighttime sleep latency but had no effect on daytime sleep (64).
Discussion
The main objective of this review was to synthesize intervention studies designed to mitigate the adverse health effects of shift work. Overall, interventions were complex and highly variable, which was reflected in the results. For example, studies of controlled light exposure used bright light, light-blocking goggles or glasses, and combinations of the two. Even within studies of intermittent bright light, patterns of exposure differed greatly with regards to timing, duration, frequency, and intensity. Therefore, it was difficult to draw direct comparisons across interventions or amongst outcomes, or to recommend one intervention to best improve the health of shift workers. We were also unable to conduct a meta-analysis to estimate magnitudes of effects for each intervention type due to study heterogeneity. Nevertheless, the main strength of this review was that all studies were conducted among participants who were engaged in night shift work. While laboratory-based studies are important for understanding the mechanisms underlying the link between shift work and adverse health outcomes, conducting workplace-based research is a key step in translating findings to real-world settings.
The aim of interventions that control light exposure is to shift circadian rhythms and subsequently promote adaptation to work at night, thereby minimizing health effects. In our review, a combination of timed bright light and light-blocking goggles appeared to promote adaptation to shift work as primarily measured by changes in sleep and melatonin. A previous review by Burgess et al (18) similarly found that timed exposure to high intensity light during night shifts and wearing goggles during the commute home can increase circadian adaptation. Although many of the studies included in the latter review were performed in simulated night shift environments, the general consistency with our review, which included more variable workplace conditions, suggests that multi-pronged interventions to control light exposure may be more effective than using bright light or light-blocking goggles alone. Due to the nature of the interventions, most studies were not blinded or randomized, resulting in a loss of quality scores for internal validity. However, scores for reporting were generally high.
Fast-forward rotating shifts tended to report more favorable results for sleep. However, findings were inconsistent for changes in shift length or start time. Shift scheduling has been attempted to improve healthy lifestyle behaviors with positive effects reported in one of the studies reviewed (41) but not in five others (39, 45, 48, 49, 53). Shift workers may be less likely to engage in regular physical activity, smoking cessation, and healthy diet, which may contribute to increased risks of adverse health outcomes (68). Objective outcomes that may be the result of improved lifestyle habits, such as low-density lipoprotein cholesterol (39); triglycerides, fasting glucose, and blood pressure (48, 49); cardiorespiratory fitness (50); and blood pressure (53), did show improvement in association with a change in shift schedule. However, improvements were not universal or consistent in magnitude across studies and studies with higher quality scores appeared to find less favorable changes. Again, because shift-scheduling changes were generally implemented across workplaces, randomization and blinding were not possible, but selection bias was of less concern because a large percentage of workers were included in each study.
Interventions directed at physical activity (56, 57) and weight loss (58, 59) improved cardiorespiratory fitness and strength (56, 57), body composition, blood pressure, and physical activity (58, 59). This suggests that lifestyle habits may not improve spontaneously among shift workers as a result of shift schedule changes, and interventions specifically targeted at improving lifestyle behaviors may be necessary.
Studies of melatonin, hypnotics, and stimulants showed mixed results, potentially due to different doses administered to workers, compliance, shift schedule variation, and other factors. Pharmacological studies were more commonly randomized controlled trials (RCT) and often double blinded, resulting in higher scores for quality. However external validity and generalizability, as well as prevalence of adverse events should be investigated in future studies. Some adverse effects were reported, including insomnia and headache from Modafinil (63, 65), and nausea, anxiety, low-back pain, and other effects from Armodafinil (64). Adverse events were also reported across other intervention types such as headaches or feelings of heat/cold in response to bright light exposure (29, 30), and difficulty scheduling social or family activities as a result of a shift schedule change (39, 41), Several studies stated that no significant adverse events resulted from the interventions (35, 36, 46, 61, 62), but most did not report adverse events at all. Since participants were not monitored for adverse health effects beyond the study period in all articles, we could not evaluate potential long-term negative health consequences of the interventions.
These findings are particularly relevant for younger rotating shift workers in the manufacturing, healthcare, and public safety sectors in Europe and North America, as these populations were most commonly represented in the studies included. Approximately one-third of shift workers studied were in manufacturing, which may partly explain the greater proportion of male compared to female shift workers in this review. Future studies should be conducted in underrepresented groups. For example, although this review identified workers between the ages of 24–58 years (most years of working life), only three studies specifically examined age effects by stratifying results by older and younger workers (40, 42, 52). The health effects of shift work may be more pronounced for older workers or those who have worked shifts for numerous years. Correspondingly, interventions to reduce the chronic health effects of shift work might have different effects on older and younger workers, warranting separate analyses.
As a secondary objective of this review, we presented aggregate and individual quality assessments in order to help identify general areas for improvement in future research. While many studies received low scores overall, and within specific sections, this may partly be attributed to the inherent limitations of the checklist selected for evaluating the quality of studies of this type. Low individual scores are not necessarily indicative of a poorly done study. The design and outcomes of these studies reflect real life workplace settings, and the information presented is useful for developing evidence informed interventions. Nevertheless, there are some changes that can be made to improve future study quality.
Reporting and external validity are areas for continued development; studies published after 2002 tended to receive higher scores (mean 17.6) than studies published before 2002 (mean 14.0), primarily due to improvements in these areas. Follow-up was quite short for many of the studies reviewed, with the longest mean follow-up observed for studies that altered shift schedules (8.2 months) and the shortest for studies of controlled light exposure (23.7 days). Since short-term changes in health outcomes may not persist in the long term, longer follow-up is needed to determine whether the interventions reviewed resulted in clinically meaningful effects on the development of chronic disease in shift workers. Sample size is another area for improvement. In this review, only three studies reported sample sizes with adequate power to detect a statistically significant difference in primary outcomes of interest (36, 58, 59, 63).
RCT are almost never feasible to implement in the workplace where an intervention affects all workers (eg, change in shift schedule) or when it is difficult to prevent contamination of study groups. Studies of shift scheduling and controlled light exposure scored particularly low on internal validity for this reason. A cluster RCT that involves randomly assigning groups of workers (eg, wards in a hospital) to an intervention may be more practical than randomizing individual workers and should be considered in future studies. Ensuring and reporting on adequate compliance, particularly in the context of controlled light exposure or behavior change interventions, is also difficult but should be urged in future studies. Lack of compliance may decrease the likelihood of finding significant health improvements and limits interpretation, reproducibility, and translation into the workplace.
Different methods used to assess similar outcomes may have also contributed to inconsistent results observed between studies of the same intervention type. For example, sleep outcomes reported using actigraphy or polysomnography (PSG), compared to sleep diaries, logs or questionnaires, more frequently found improvements in sleep. Of the five studies reporting both subjective and objective measures, two showed improvements only in objective measures (37, 60) two showed improvements only in subjective measures (24, 64), while one showed improvements in both (40). While logs or questionnaires may have lacked adequate sensitivity to detect sleep pattern changes, actigraphy or PSG were limited by technical issues or poor compliance. Future studies should consider using both objective and self-reported measures to enhance validity, and standard methods for measuring sleep-related outcomes.
We excluded self-reported sleepiness as an outcome, as it is more closely related to workplace safety than chronic disease risk (36, 43). However, of the included interventions that reported on sleepiness, findings were aligned with sleep quality and quantity results (23, 24, 33, 36, 42, 53, 60, 64). One exception was a study of Modafinil, which significantly reduced sleepiness during the commute home from a night shift but had no effects on sleep (63). While sleepiness during commuting is an important problem for shift workers, this indicator is not related to chronic disease risk (ie, the focus of our review). We also excluded absenteeism as an outcome, as it is most closely related to productivity and work-related outcomes. However, it is possible that disease-specific sickness absence could provide an indication of chronic disease diagnosis and severity and should be considered in future studies.
Following our systematic search, we briefly scanned the literature for articles published between 14 August 2012 and 1 May 2014, and identified four that may have met our inclusion criteria (69–72). Future reviews that integrate newer studies would be a valuable addition to the state of the science as synthesized in this paper.
Evaluating preventive strategies among shift workers is a relatively new and evolving area of research. This critical review highlights the range of practical interventions conducted in “real life” workplace settings. Previous reviews have been limited by considering either a single intervention type or outcome, or by including studies conducted in laboratory settings, worksite and home-based interventions, and by including both shift workers and healthy volunteers. The scope of our search on multiple databases enabled us to include 38 interventions representing four general intervention types. Our search was rigorous, spanning three large databases for all years up to 13 August 2012. This allowed us to minimize publication bias and identify most of the relevant studies for the aim of this review. This review also illuminates important gaps in shift work intervention research.
Combinations of intervention types and personalized interventions offer promising ways to improve the health of shift workers but were not identified in our search. Comprehensive, evidence-based approaches that include best practices in shift scheduling, a range of options to control exposure to light and dark, support for physical activity and healthy eating, as well as pharmacological agents, may be the best ways to improve health. There is also a need to develop and test novel approaches, like social support, possibly using new technologies such as smart phones to help with sleep or other adverse effects.
As shift work becomes increasingly prevalent, relevant and high-quality research conducted on large numbers of shift workers in their normal working conditions and workplaces to test the effectiveness of different interventions is required. There is no “one size fits all” solution, and individual shift workers may have different responses to interventions as the result of chronobiology, personal preferences that affect compliance, or other factors that remain to be assessed. Intervention research should account for potential biases and other lifestyle, work, and environmental confounding factors that might be related to shift work and chronic disease. Innovative, evidence-based prevention efforts should be developed and evaluated to simultaneously meet the unique health needs of shift workers and the mandates of the organizations and industries in which they work. This is a promising area with many potential areas for further investigation.