Parkinson’s disease is a degenerative disorder affecting about 1% of the population >65 years of age (1). Defective transmission of the neurotransmitter dopamine is central in the pathophysiologic process of this disease. The transmembrane protein α-synuclein is essential for release of dopamine from the presynaptic neurons (2, 3). If α-synuclein becomes misfolded, the protein will instead be deposited in inclusion bodies, in this case Lewy bodies, and the dopamine neuron will successively degenerate (4). The diagnosis of Parkinson’s disease is based on a symptom triad, a progressive course, and a positive response to treatment with L-dopa (5). Misfolding of α-synuclein can be induced by exposure to both pesticides and metals (1). Although other pathophysiologic mechanisms (not primarily involving α-synuclein) can also be trigged by exposures, the hallmark of Parkinson’s disease is that the damage involves the presynaptic dopamine neurons (4).
Exposure to metals can sometimes induce clinical pictures similar to Parkinson’s disease, most commonly manganism (6), but in these cases the neuron damage is instead situated postsynaptically. The diagnosis is Parkinsonism or Parkinson-plus, the disease is not progressive after exposure is terminated, and the symptoms are not alleviated by treatment with L-dopa. In epidemiologic studies, it is thus very important to separate Parkinson’s disease from similar disorders that involve different pathophysiologic mechanisms.
Six systematic literature reviews and meta-analyses have been published on associations between Parkinson’s disease and occupational exposures (7–12). The most recent and also most comprehensive meta-analysis included 19 publications on occupational exposure to pesticides, and showed a weighted relative risk (RR) of 1.49 with a 95% confidence interval (95% CI) of 1.34–1.66 (7). Additional meta-analysis was performed on the separate pesticide groups, with herbicides having an RR of 1.52 (95% CI 0.99–2.33), insecticides of 1.29 (95% CI 0.69–2.41), and fungicides of 1.10 (95% CI 0.62–1.92). The summary RR for exposure to insecticides and herbicides showed a statistically significant association with Parkinson’s disease, especially for occupational exposure. The most elevated weighted RR were found for exposure to the chemical agents organochlorine (RR 1.46, 95% CI 0.86–2.47) and paraquat (RR 1.38, 95% CI 0.72–2.66) based on four and six studies, respectively.
A second meta-analysis on exposure to pesticides involved 19 studies published between 1989 and 1999. The weighted RR was 1.94 (95% CI 1.49–2.53) (11). Another meta-analysis on exposure to pesticides was based on cohort studies only and gave an RR of 1.28 (95% CI 1.03–1.59) (9). Exposure to hydrocarbons was addressed in one meta-analysis based on 13 studies; the weighted RR was 1.36 (95% CI 1.13–1.63) (8). A meta-analysis which included 13 studies exploring the association with exposure to welding and manganese gave a pooled RR of 0.86 (95% CI 0.80–0.92) (10). Another comprehensive meta-analysis showed that there was no association between exposure to electromagnetic fields and Parkinson’s disease (RR 0.97, 95% CI 0.91–1.03) (12).
The abovementioned meta-analyses included all relevant publications, irrespective of whether the studies suffered from major weaknesses in design or ascertainment of diagnosis, were biased by missing data, or used insufficient statistical methods. Such faultiness might have hampered the weighted risk ratios, and so there is a need for meta-analyses based only on publications fulfilling high scientific standards.
One of the biggest public insurance companies on the Swedish labor market (AFA Insurance) needed a scientifically based standard for evaluating work-related disease and commissioned us to perform an updated foundation for decisions both regarding prevention and compensation of damages. We considered all work related exposures with regard to Parkinson’s disease and conducted a systematic review of the published literature, scrutinized relevant publications, and carried out meta-analyses using only studies that fulfilled good scientific standards. Our report was published in 2014 in a Swedish peer-reviewed series of publications (gupea.ub.gu.se/handle/2077/35819). The present publication aims to update our previous review and meta-analyses and make the results available for an international public.
Methods
Literature search
We identified relevant published articles using bibliographic search engines in PubMed, Embase, and Arbline prior to 1 February 2016. Our search criteria were MeSH terms for study design (cohort, epidemiol*, epidemiologic studies) in combination with MeSH terms for exposure (employment, workplace, professions, career, career choice, job, occupations, employment, occupational health, occupational medicine, occupational exposure, occupational injuries, occupational diseases, electromagnetic field) in combination with any of the MeSH terms for the disease of interest (Parkinson, Parkinson’s disease, Parkinsonism). This search produced 240 articles. After we scrutinized the titles and/or abstracts, excluded a few duplicates, and included only articles based on original data on exposures related to occupation, 132 potentially relevant articles remained.
Quality classification
We scrutinized all relevant publications according to the MOOSE guidelines for meta-analyses and systematic reviews of observational studies (13). We considered selection bias and “falling off” (15), as well as occurrence of dose–response effects (16) and used a system for grading observational epidemiologic articles into a global score I-V proposed by Armon (14). Based on these documents, we constructed a decision protocol involving the following categories: diagnosis, exposure, study group (selection, controls, missing data), methods and analyses, Armon global score, funding, and exposures. The Armon checklist and our decision protocol is presented in an appendix (www.sjweh.fi/index.php?page=data-repository). The quality of the diagnosis was graded as: 1=the UK Parkinson’s Disease Society Brain Bank criteria (5) were applied, 2=diagnosis from a hospital (as an in-patient), 3=diagnosis from a GP (also including mortality registers), and 4=Parkinson’s disease and Parkinsonism were not separated in the analyses. The other categories were graded 1=good, 2=sufficient, 3=uncertain/insufficient, or 4=unacceptable. Sometimes a category was graded in between, and thus was given an interval, for example 2–3. The reason for this was usually lack of sufficient information to carry out a more specific categorization.
Prerequisites for accepting a publication to fulfil good scientific standards (Armon global score II or III) were: (i) diagnosis had to achieve 1–3 score. All the other categories should involve 1–2 as a single score or be included in the interval 2–3. Articles not qualifying for global score II–III were impaired by serious weaknesses (Armon score IV) or should be ignored (Armon score V). None of the publications fulfilled Armon global score I, which almost requires an experimental design.
Only studies fulfilling good scientific standards (Armon class II or III) were used in our meta-analyses (17–64); see table 1. Relevant publications (65–121) not fulfilling good scientific standards are summarized in table 2.
Table 1
Publications fulfilling good scientific standards (Armon global score II or III) defined in the appendix (www.sjweh.fi/index.php?page=data-repository). Diagnosis was graded at least score 3 (diagnosis from neurologist, in-patient care, mortality register). The other categories involved at least a single score 2 or included in the interval 2–3 (1=good, 2=sufficient, 3=uncertain/insufficient). [PA=patient association; PU=public; EMF=electromagnetic fields.]
| Publication | Diagnosis | Exposure | Study group (selection, controls, missing data) | Methods and analysis | Armon global quality score | Funding | Exposures |
|---|---|---|---|---|---|---|---|
| Ascherio et al, 2006 (17) | 1 | 2 | 2 | 1 | II | PU | Pesticides |
| Brighina et al, 2008 (18) | 2 | 1 | 2 | 2 | II | Industry | Pesticides |
| Butterfield et al, 1993 (19) | 2 | 1 | 3 | 2 | III | PU | Pesticides + other chemicals |
| Chen et al, 2006 (20) | 2 | 2 | 2 | 2 | II | ? | Night shift work |
| Conn et al, 2006 (21) | 2 | 1 | 3 | 2–3 | III | PA, PU | Lead |
| Dutheil et al, 2010 (22) | 2 | 1 | 2 | 1 | II | PU | Pesticides |
| Elbaz et al, 2009 (23) | 1 | 1 | 2 | 1 | II | PU | Pesticides |
| Feldman et al, 2011 (24) | 2–3 | 2 | 1 | 2 | III | Industry | Pesticides, metals, welding |
| Feychting et al, 2003 (25) | 3 | 2–3 | 1 | 1 | III | PU | Occupation (EMF) |
| Firestone et al, 2010 (26) | 2 | 2 | 2–3 | 2 | III | PU | Occupation (pesticides, welding, metals) |
| Fong et al, 2007 (27) | 1 | 2–3 | 2–3 | 2 | III | ? | Pesticides |
| Fored et al, 2006 (28) | 2 | 2–3 | 1 | 2 | III | PU | Occupation (welding) |
| Frigerio et al, 2005 (29) | 1 | 2–3 | 2 | 2 | III | PU | Occupation (metals) |
| Fryzek et al, 2005 (30) | 2–3 | 2 | 2–3 | 2 | III | Industry | Welding |
| Galanaud et al, 2005 (31) | 1 | 1 | 2–3 | 1–2 | III | PU | Pesticides |
| Gorell et al, 1997 (32) | 2 | 2 | 3 | 2 | III | PA, PU | Metals |
| Gorell et al, 1998 (33) | 2 | 2 | 3 | 2 | III | PA, PU | Pesticides + other chemicals |
| Hakansson et al, 2003 (34) | 3 | 2–3 | 2 | 1 | III | Industry | Occupation (EMF) |
| Hancock et al, 2008 (35) | 1 | 2 | 2–3 | 1 | III | PU | Pesticides |
| Harris et al, 2012 (36) | 2 | 1 | 2–3 | 2 | III | PU | Vibrations |
| Harris et al, 2012 (37) | 2 | 2 | 2–3 | 2 | III | PU | Animals |
| Hertzman et al, 1994 (38) | 1 | 1 | 2 | 2–3 | III | PU | Pesticides |
| Johansen et al, 1998 (39) | 3 | 2 | 2 | 2–3 | III | PU, Industry | Occupation (EMF) |
| Johansen et al, 2000 (40) | 2 | 2 | 2 | 2 | III | PU, Industry | Occupation (EMF) |
| Kamel et al, 2007 (41) | 3 | 2 | 2 | 2 | III | PU | Pesticides |
| Kenborg et al, 2011 (42) | 2–3 | 2–3 | 1 | 1 | III | PU | Occupation (out-door work) |
| Kenborg et al, 2012 (43) | 3 | 2 | 2 | 2 | III | ? | Occupation (welding) |
| Kuopio et al, 1999 (44) | 1 | 2 | 2 | 2–3 | III | PA | Pesticides + animals |
| Kwon et al, 2013 (45) | 1 | 2 | 2 | 2 | II | PU | Out-door work |
| Liew et al, 2014 (46) | 2 | 1 | 2 | 1 | II | PU, PA | Pesticides |
| Manthripragada et al, 2010 (47) | 1 | 1 | 2 | 2 | II | PA | Pesticides |
| Noonan et al, 2002 (48) | 3 | 2–3 | 2 | 1–2 | III | ? | Occupation (EMF) |
| Park et al, 2005 (49) | 3 | 2–3 | 2 | 2 | III | PU | Occupation (EMF, pesticides, welding) |
| Petrovitch et al, 2002 (50) | 1 | 2 | 1 | 1 | II | PU | Pesticides |
| Roosli et al, 2007 (51) | 3 | 1 | 2 | 1 | III | PU | Occupation (EMF) |
| Rugbjerg et al, 2011 (52) | 2 | 2 | 2–3 | 2 | III | PU | Pesticides |
| Savitz et al, 1998 (53) | 3 | 2–3 | 2 | 2 | III | Industry | Occupation (EMF) |
| Savitz et al, 1998 (54) | 3 | 2–3 | 2 | 2 | III | ? | Occupation (work with electricity) |
| Seidler et al, 1996 (55) | 2 | 1 | 2 | 2 | II | PU | Pesticides + other chemicals |
| Semchuk et al, 1992 (56) | 2 | 1 | 2 | 2 | II | PU | Pesticides + other chemicals |
| Sorahan et al, 2007 (57) | 3 | 1 | 2 | 2 | III | ? | Power station workers (EMF) |
| Stampfer et al, 2009 (58) | 3 | 2–3 | 2 | 2 | III | Industry | Occupation (welding) |
| Tanner et al, 2011 (59) | 2 | 2 | 2 | 2 | II | Industry | Pesticides |
| Tanner et al, 2009 (60) | 1 | 2 | 2–3 | 2–3 | III | Industry | Pesticides + welding |
| Tuchsen et al, 2000 (61) | 2 | 2–3 | 2 | 2 | III | PU | Occupation (agricultural work) |
| Valdes et al, 2014 (62) | 2–3 | 2 | 2–3 | 2 | III | PU, PA | Occupation (work complexity) |
| Wang et al, 2011 (63) | 1 | 1 | 2–3 | 1 | III | PU | Pesticides |
| Wastensson et al, 2006 (64) | 2 | 1 | 2 | 2–3 | III | PU | Pesticides |
Table 2
Publications not fulfilling good scientific standards (Armon global score IV or V) defined in the Appendix (www.sjweh.fi/index.php?page=data-repository). Diagnosis was graded as 4 (Parkinson’s disease and Parkinsonism were not separated) or any other category involved a single score 3 or 4 (3=uncertain/insufficient, 4=unacceptable). [PA=patient association; PU=public; EMF=electromagnetic fields.]
| Publication | Diagnosis | Exposure | Study group: selection, controls, missing data | Methods and analysis | Global quality score according to Armon | Funding | Exposures |
|---|---|---|---|---|---|---|---|
| Baldereschi et al, 2003 (65) | 1 | 3 | 2–3 | 3 | IV | PU | Pesticides |
| Baldi et al, 2003 (66) | 2 | 3 | 2–3 | 3 | IV | PU | Occupations (pesticides) |
| Baldi et al, 2003 (67) | 2 | 2–3 | 3 | 1 | IV | PU | Occupations (pesticides) |
| Behari et al, 2001 (68) | 2 | 2–3 | 3–4 | 2 | IV | PU | Pesticides |
| Brouwer et al, 2015 (69) | 3 | 2–3 | 3 | 2 | IV | PU | Occupations (pesticides) |
| Chan et al, 1998 (70) | 1 | 2–3 | 3 | 3–4 | IV | Industry, PA, PU | Pesticides |
| Chatuverdi et al, 1995 (71) | 3 | 3 | 3 | 3–4 | IV | PU | Miscellaneous |
| Costello et al, 2009 (72) | 1 | 3–4 | 3–4 | 2 | IV | PU | Pesticides |
| Das et al, 2011 (73) | 2 | 2–3 | 3 | 4 | V | PU | Pesticides |
| Dhillon et al, 2008 (74) | 1 | 2–3 | 4 | 4 | V | PU | Pesticides + metals |
| Dick et al, 2007 (75) | 3 | 2–3 | 3 | 2 | IV | PU | Occupation (pesticides) |
| Dick et al, 2007 (76) | 3 | 2–3 | 3 | 3 | IV | PU | Occupation (pesticides) |
| Duzcan et al, 2003 (77) | 3 | 3 | 3 | 3–4 | IV | ? | Pesticides |
| Fall et al, 1999 (78) | 1 | 3–4 | 2 | 2 | IV | PU | Pesticides |
| Frigerio et al, 2006 (79) | 2 | 2 | 3 | 2 | IV | PU | Pesticides + other chemicals |
| Goldman et al, 2005 (80) | 2 | 3 | 4 | 3 | V | Industry | Occupation |
| Gorell et al, 2004 (81) | 2 | 2 | 3 | 3–4 | IV | PA, PU | Pesticides + other chemicals + metals |
| Herishanu et al, 2001 (82) | 2 | 2–3 | 3 | 3–4 | IV | PU | Pesticides + other chemicals + metals |
| Hertzman et al, 1990 (83) | 1 | 2–3 | 2–3 | 3–4 | IV | PU | Pesticides |
| Hofmann et al, 2006 (84) | 3 | 3 | 3 | 2–3 | IV | PU | Occupation (pesticides) |
| Hubble et al, 1993 (85) | 2 | 2–3 | 3–4 | 3–4 | IV | ? | Pesticides |
| Kenborg et al, 2012 (86) | 2 | 3–4 | 2 | 3 | IV | ? | Garden work |
| Kirkey et al, 2001 (87) | 2 | 2 | 3 | 3–4 | IV | ? | Occupation (agricultural work) |
| Lehman et al, 2012 (88) | 3 | 1 | 2 | 3 | IV | PU | Professional American Football |
| Li et al, 2009 (89) | 2 | 2–3 | 3 | 2–3 | IV | PU | Occupation |
| Liou et al, 1997 (90) | 2 | 2–3 | 4 | 2 | V | PU | Pesticides + other chemicals + metals |
| Marsh et al, 2006 (91) | 4 | 2 | 2–3 | 2 | V | Industry | Company register (welding) |
| McCann et al, 1998 (92) | 2 | 3 | 3 | 3–4 | IV | PU | Pesticides |
| McDonnell et al, 2003 (93) | 3 | 2 | 3 | 2 | IV | ? | Pension fund register (pesticides + other chemicals) |
| Menegon et al, 1998 (94) | 2 | 2 | 3–4 | 2 | IV | PU | Pesticides |
| Ngim et al, 1989 (95) | 2 | 2 | 4 | 4 | V | PU | Metals |
| Nuti et al, 2004 (96) | 3 | 2 | 3 | 3 | IV | ? | Pesticides |
| Ohlson et al, 1981 (97) | 2 | 2–3 | 2–3 | 3 | IV | PU | Pesticides + other chemicals + metals |
| PArk et al, 2006 (98) | 2 | 2–3 | 3 | 3–4 | IV | PU | Company register (metals) |
| PArk et al, 2005 (99) | 2 | 2 | 3 | 3 | IV | ? | Occupation |
| PArk et al, 2005 (100) | 2 | 2 | 3 | 3–4 | IV | PU | Occupation |
| Peretz et al, 2005 (101) | 3 | 2–3 | 3 | 2–3 | IV | PU | Occupation (anesthesiology versus internal medicine) |
| Petersen et al, 2008 (102) | 1 | 2 | 3 | 3–4 | IV | PU, PA | Pesticides, heavy metals |
| Racette et al, 2005 (103) | 2 | 2–3 | 4 | 3–4 | V | PA | Welding |
| Rocca et al, 1996 (104) | 2 | 2–3 | 3 | 3 | IV | PU | Occupation |
| Rybicki et al, 1999 (105) | 2 | 2 | 3 | 3 | IV | PU, PA | Metals |
| Schulte et al, 1996 (106) | 3 | 3 | 2–3 | 3–4 | IV | ? | Occupation |
| Smargiassi et al, 1998 (107) | 2 | 2 | 3 | 3–4 | IV | PU | Chemicals |
| Steenland et al, 2013 (108) | 2 | 2–3 | 3 | 3–4 | IV | PU | Pesticides |
| Stern et al, 1991 (109) | 2 | 2–3 | 4 | 2 | V | Industry | Pesticides |
| Tanaka et al, 2011 (110) | 2 | 3 | 3–4 | 2–3 | IV | PU | Pesticides + other chemicals |
| Tomeson et al, 2011 (111) | 3 | 2 | 3 | 3 | IV | Industry | Pesticides |
| Tsui et al, (112) 1999 | 2 | 3 | 4 | 3 | V | PA | Occupation |
| van der Mark et al, 2014 (113) | 4 | 1 | 3 | 2 | V | PA | Pesticides |
| van der Mark et al, 2015 (114) | 4 | 2–3 | 3 | 1 | V | PA | Electromagnetic fields |
| Wechsler et al, 1991 (115) | 2 | 3 | 3 | 4 | V | ? | Chemicals |
| Werneck et al, 1999 (116) | 1 | 2–3 | 3–4 | 3 | IV | ? | Pesticides + other chemicals |
| Vlajinac et al, 2010 (117) | 2 | 2–3 | 3 | 3–4 | IV | PU | Chemicals + metals |
| Wong et al, 1991 (118) | 2 | 2–3 | 3–4 | 4 | V | ? | Pesticides + other chemicals |
| Wright et al, 2005 (119) | 4 | 2–3 | 3 | 3 | V | PU | Pesticides |
| Yang et al, 2015 (120) | 3 | 2 | 3–4 | 1 | IV | PU | Physical activity |
| Yesalis et al, 1985 (121) | 4 | 2–3 | 3 | 3 | V | PU | Occupation |
Statistical analysis
Risk estimates from the selected studies are reported as RR, as the outcome is rare, and so odds ratios (OR) and hazard ratios (HR) can be considered equivalent to the RR. When unadjusted as well as multivariable-adjusted risk estimates were reported, we only considered the adjusted estimates. Studies which reported stratified estimates for sex were considered as separate studies, and included in the sex-stratified analysis. In the overall table, only the combined estimate was used, with sex included as a confounder in a multivariable analysis. In a few sex-stratified studies where no combined estimate was available, only the estimate for males was included in the overall table since the female stratum in general had very few cases and its effect on the combined estimate could only be of a very small magnitude. For studies that reported more than one risk estimate per exposure, we considered the size of the study group and the number of exposed cases, and where an extremely small number of individuals were considered this estimate was not included in analogy with the discussion above on sex-stratified analysis. In order to have comparable study populations in the meta-analyses, we only included studies covering all ages of the working population.
We examined the fixed- and random-effects models by considering statistical heterogeneity. To this end, we used the I2 statistic where the recommended cut-offs of 25%, 50%, and 75% degrees of heterogeneity were considered. We also used a meta-regression approach to stratify on study characteristics, selected a priori and evaluate the significance of the stratification variable. The I2 criterion was also applied to examine heterogeneity for each examined strata. As both these tests indicated a random effects model as the most appropriate choice in almost all studies, the results are reported with random-effects estimates. The weights used for pooling the risk estimates were equal to the inverse-variance weighting. We also performed leave-one-out analysis for each study to check the influence of each study on the combined estimate.
Publication bias was analyzed by inspection of the funnel plot, in which the estimates of RR should be distributed symmetrically around the weighted RR unless the publication was affected by bias. The Beg & Mazumdar (122) rank correlation test was used to supplement the interpretation of the funnel plot.
Results
Exposure to pesticides
The most extensively studied aspect was the association between Parkinson’s disease and exposure to pesticides, which was examined in 25 studies fulfilling good scientific standards (table 1) and 34 studies of lower quality (table 2). The study with the highest effect size (RR) included 63 patients diagnosed with Parkinson’s disease before the age of 50. The RR was statistically significantly elevated for exposure to insecticide (RR 5.75) and herbicide (RR 3.22) (19). Another study covering 351 cases reported exposure to three specified insecticides, but no weighted RR for pesticides was presented (47).
The remaining 23 studies fulfilling good scientific standards reported RR that could be included in the meta-analyses (see figure 1). The weighted RR was 1.67 with a 95% CI 1.42–1.97. In the Gorell study, separate RR were presented for all the groups of pesticides (insecticide, herbicide, and fungicides) (33). Separate meta-analyses were thus performed for each group of pesticides. For insecticides, we chose the “worst case” exposure scenario by selecting the substance with the highest weighted RR for the calculations presented in figure 1. Using the risk estimates for herbicides or fungicides entailed only slightly different RR (1.66 and 1.62, respectively).
Figure 1
Forest plot for studies assessing the association between Parkinson’s disease and occupational exposure to pesticides. Results for men only are indicated by M; otherwise the results concern both sexes. Random effect models were used, with stratification of study design. Heterogeneity was tested by the I2 statistic, with P<0.05 indicating rejection of homogeneity.
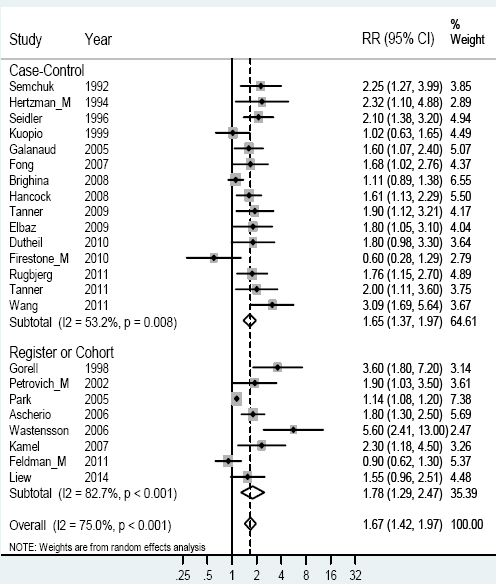
The study reporting a low RR for association between Parkinson’s disease and exposure was based only on twelve men, and hampered by missing data. Five men were exposed to parathion, and the RR was 5.8 with a wide confidence interval (26).Our validation test using leave-one-out estimation showed a fairly stable estimate, with RR of 1.62–1.74 and only minor variations in heterogeneity.
The heterogeneity was most pronounced in the cohort and register studies, as shown by higher square percentages in figure 1. In order to study factors exerting influence on the heterogeneity in the results, we carried out further meta-analyses based on different stratification factors. Stratifying on source of funding (see table 1) with public funding versus other funding gave RR of 1.90 (95% CI 1.58–2.28) and 1.38 (95% CI 1.13–1.67) respectively, and the heterogeneity still remained. Stratifying by quality (grading II against II–III and III) resulted in RR of 1.88 (95% CI 1.52–2.33) for the higher grading and 1.61 (95% CI 1.34–1.92) for the lower grading. The higher grading had almost no heterogeneity (I2 =0.0%), but the lower grading showed heterogeneity (I2=75.1%).
Seven of the studies reported results for men (17, 22, 24, 26, 38, 46, 50), and three reported results for women (17, 26, 38). Women showed a higher weighted RR of 1.80 (95% CI 1.03–3.15) with small heterogeneity, while men showed an RR of 1.50 (95% CI 1.05–2.16) based on a more profound heterogeneity. This heterogeneity was still high if the analysis for men was restricted to only those three studies that reported results for women [RR 1.41 (95% CI 0.70–2.82)].
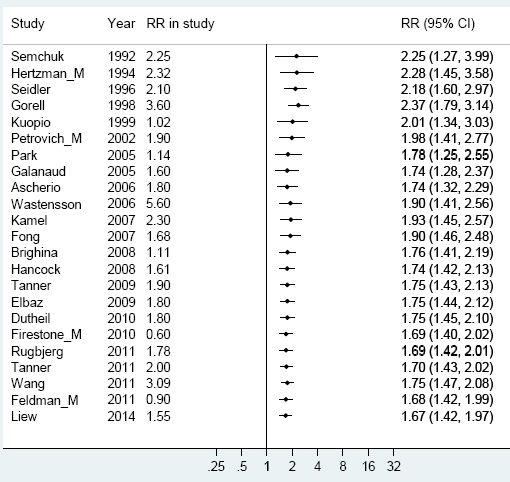
A meta-analysis over the period 1990–2011, in which the results of earlier studies were gradually cumulated, showed a successive reduction of RR from 2.25 to 1.67 (figure 2).
Figure 2
Cumulative meta-analyses on the association between Parkinson’s disease and pesticide exposure with the pooled estimate updated for every new study, year by year. [RR=relative risk.]
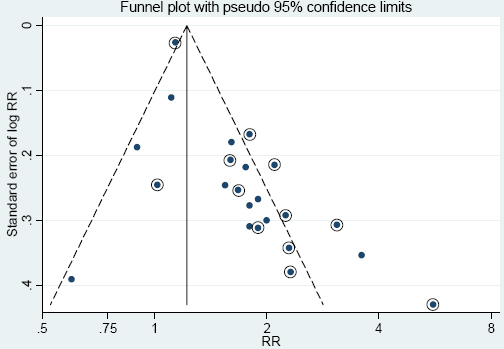
A funnel plot was constructed in order to evaluate publication bias. The asymmetry in the funnel plot shown in figure 3 indicates publication bias. The bias was most evident for smaller studies, especially those published before 2008 (circled dots), and supported by Begg’s test for bias. For the period 1994–2007, the weighted RR was 1.90 and Begg’s test gave P=0.075. However, for the later period of 2008–2014, the weighted RR was 1.50 (95% CI 1.20–1.88) and Begg’s test showed no publication bias (P=0.243).
Figure 3
Funnel plot for the RR estimates of the association between Parkinson’s disease and pesticide exposure in the 23 different studies of figure 1. Circled dots represent studies published before 2008.
Exposure to metals
Exposure to welding was addressed in eight studies (24, 26, 28, 30, 43, 49, 58, 60), giving a weighted RR of 0.85 (95% CI 0.82–0.89), and exposure to metals in other forms was addressed in five studies (21, 24, 26, 29, 32), giving a weighted RR of 0.98 (95% CI 0.53–1.81). There was no indication of publication bias.
Electromagnetic fields and work with electricity
Exposure to electromagnetic fields was addressed in two case–control studies (48, 53) and eight register/cohort studies (25, 34, 39, 40, 49, 51, 54, 57). The weighted RR was 1.07 (95% CI 0.97–1.19).
As some heterogeneity was present, different stratifications were tested. Stratifying by design gave RR of 1.33 (95% CI 0.85–2.09) for studies with a case–control design and 1.02 (95% CI 0.90–1.16) for register and cohort studies, still with some degree of heterogeneity. Stratification by sex was not possible since there was only one study that specifically considered women (25). Stratification by quality gave RR of 1.31 (95% CI 0.97–1.78) for studies of class II and 1.05 (95% CI 0.97–1.14) for class III. Stratification by funding showed that studies with public funding had reduced heterogeneity and an RR of 0.99 (95% CI 0.82–1.18). Overall, there was no indication of publication bias as judged from the funnel plot, and Begg’s test gave a P-value of 0.70.
Other exposures
Two studies fulfilling good scientific standards indicated a lower risk for Parkinson’s disease among persons on night shift work (20) and those working outdoors (40), respectively.
Three publications examined whether certain occupations involved an increased risk for Parkinson’s disease (29, 49, 61). The first of these had a study population consisting of incident cases from hospital registers and used interviews to gather data on occupation; this revealed an increased risk of Parkinson’s disease for physicians and people with higher education (29). The second used registers for the whole Danish population in hospital care to show that Parkinson’s disease was more common among those who had worked in agriculture/horticulture (61). Finally, matching of computer files for mortality and occupation in the USA indicated a higher prevalence of Parkinson’s disease among persons in biological science, teachers, and employees in churches (49).
A Canadian study based on 400 cases of Parkinson’s disease in people aged 40–69 years indicated that ever having been exposed to whole body vibrations was inversely associated with the disease (36), while another study showed statistically elevated RR for exposure to cats and cattle (37). Only one epidemiological study of good scientific standards addressed occupational complexity, finding that high-complexity work with data and people was related to increased risk of Parkinson’s disease, particularly among men. The attenuation of risk observed in the twin-pair stratified analyses suggests that the association may partly be explained by familial factors (62).
Discussion
Weighted risk estimates based on 23 scientifically high quality epidemiologic publications show that the risk of Parkinson’s disease is elevated by >50% after exposure to pesticides. Publication bias might have had some influence on the results, but can only partly explain the elevated risk. Studies published after 2007 showed less publication bias and a somewhat lower risk estimate, but the elevation was still in the vicinity of 50%.
One general limitation of meta-analyses is that the calculations can only be based on published data and will reflect any inherent weaknesses of design in the studies included. Furthermore, all previously published meta-analyses on Parkinson’s disease have been based on all relevant publications identified, irrespective of the quality of the study design.
One strength of our study is that the meta-analyses were based on a systematic literature review including only studies fulfilling scientifically high quality standards. Based on Armon’s detailed checklist (14), we established a convenient protocol (referred to in tables 1 and 2) for scrutinizing publications. Studies were only included if the diagnosis was appropriate (score 1–3), and so studies mixing Parkinson’s disease patients with Parkinsonism patients were excluded even if they fulfilled good scientific standards in all other respects (91, 113, 114). The latter disorder is caused by quite different pathophysiological mechanisms and would thus be related to different occupational exposures and pathogenesis.
Regarding exposure (14), we made sure that only exposures occurring some years before the start of overt disease were considered and that methods of gathering information on exposure were the same for both affected and healthy individuals. The most desirable aspect was quantification of exposure by experts (occupational hygienists) blinded to the health status of the participants. It was also necessary to take recall bias into consideration. Several study groups were hampered by bias caused by missing data (low response rate) from cases or controls or inappropriate matching of controls. Regarding methods and analyses, attention was paid to sources of bias and confounding as well as use of appropriate statistical methods. Based on all these parameters, we each individually assessed a global score (14) for every publication; if our scores were divergent, we re-examined the publication and after discussion found consensus. There is always room for a reader’s own discretion when judging a publication, and it is possible that other authors would have categorized some of these publications into global score III instead of global score IV, and vice versa. However, when we got used to the protocol for grading different aspects of studies the consensus between us became almost complete.
Another strength of our meta-analyses is that we focused heavily on finding all possible sources of bias, using stratification of data with regard to possible confounders such as study design, gender, and funding. We also looked for publication bias using both funnel plots and a test for publication bias.
Our cumulative meta-analyses (figure 2) illustrate that the influence from earlier studies on pesticides declined when later studies with lower RR were added. Figure 2 can also be used to read the state of evidence with regard to risk rate for each year over the period, indicating either a tendency toward publication bias in earlier years or an effect of increased protection against exposure over time.
The risk estimate for pesticides found in our meta-analysis was of the same magnitude as in three other published meta-analyses (7, 9, 11). In some studies, separate risk estimates are presented for different types of pesticides (18, 19, 22, 23, 26, 38, 44, 55, 56, 59, 60); the risk estimates are somewhat higher for insecticides and herbicides compared to fungicides. Pesticides are designed to interfere with cellular mechanisms, and so it is not surprising that exposure to any pesticides seems to exert an increased risk for Parkinson’s disease. Some mechanisms are discussed in two publications by Tanner (59, 60).
Several publications have addressed electromagnetic fields and welding as possible risk factors for Parkinson’s disease, but in line with our meta-analyses, no associations have been found (10, 12). Instead, a slightly but statistically significantly reduced risk estimate was found regarding exposure to welding. This might be due to the healthy worker effect, caused by selection of healthier people into highly skilled physical work. Another possible explanation is the biological phenomenon of hormesis, which implies that exposure to low levels of toxic substances can induce enhancement of protective biological mechanisms (123).
Some few other studies fulfilling good scientific standards addressed other exposures, some statistically significant associated to Parkinson’s disease. However, results from single studies do not convey the truth. The exposure has to be explored repeatedly in other studies preferably of different designs. Thus single study results should only be regarded as hypothesis generating in relation to exposures, which might be worth to investigate further.
Earlier studies have shown that a family history of neurodegenerative diseases implies an increased risk for development of Parkinson’s disease (124). Such heredity has an especially high impact for people with onset of Parkinson’s disease before age 50 (125). Smoking involves an increased risk for almost all diseases; but in the case of Parkinson’s disease, over 50 studies done over the last half century have consistently demonstrated a reduced prevalence of Parkinson’s disease among smokers compared to never-smokers (126). Several neuroprotective mechanisms are discussed and associated both with combustion products and nicotine itself (126).
Concluding remarks
Meta-analyses only on studies fulfilling good scientific standards showed that exposure to any pesticide involves ≥50% increased risk for developing Parkinson’s disease. Using studies of high scientific quality resulted in less heterogeneity in the meta-analyses and also higher risk estimates. Other occupational exposures such as metals and electromagnetic fields did not bring about increased risk.
Before deciding to involve publications into meta-analyses we would recommend procedures similar to the one presented here for grading scientific quality. This would diminish variance and result in more stable risk estimates.



