Testicular cancer is the most common cancer among 15−44-year-old men in countries with high or very high Human Development Index (HDI) scores (1). There are large ethnic and geographic variations within these countries, eg, men of European descent have higher incidence compared to men of African or Asian origin in the US (2). Historically, there were also substantial differences in incidence rates among the Nordic countries [age-standardized rates (ASR World) per 100 000 in 1980: Denmark 8.5, Finland 1.2, Norway 5.9, Sweden 3.9] but the incidence rates have become more similar over the last ten years (ASR World per 100 000 in 2010–2014: Denmark 9.9, Finland 5.8, Norway 11.3 and Sweden 7.1) (3). Such geographical variation suggests that environmental exposures are likely to play a major role, further supported by migration studies showing that 1st generation migrants have similar risk as men in their countries of origin, while their sons (2nd generation migrants) attained a risk similar to men in their new home country (4, 5).
The majority (>95%) of testicular cancers are testicular germ cell tumors (TGCT) including seminomas and non-seminomas (6). Factors that have been strongly associated with TGCT include congenital malformations (cryptorchidism and hypospadias), and family history of TGCT (7). Also, TGCT in young adults are preceded by germ cell neoplasia in situ (GCNIS) and of different origin than rarer non-GCNIS-related TGCT ie, yolk sac tumors and immature teratomas occurring during childhood, and spermatocytic seminomas affecting mostly men over 50 years of age and that are not of fetal origin (8). Prenatal and perinatal risk factors are plausible causative candidates because GCNIS-related TGCT occurs relatively early in life and is associated with other male reproductive disorders starting during fetal life (9). Experimental studies have shown that prenatal exposure to endocrine disrupting chemicals affects the development of reproductive organs in male offspring (10, 11). In humans, some epidemiologic have suggested an association between intrauterine exposure to endocrine disrupting chemicals with male reproductive disorders, yet the evidence remains limited (12–16).
Organic solvents, such as toluene, benzene, perchloroethylene and trichloroethylene are found in a variety of industrial products such as pesticides, resins, glues, paint thinners, and degreasers (eg, in the metal industry) and serve as raw materials or intermediate in the production of other chemicals. Within the International Agency for Research on Cancer’s (IARC) monograph program on the evaluation of carcinogenic risks to humans, benzene (mainly for leukemia) and trichloroethylene (kidney cancer) have been classified as carcinogenic to humans; and methylene chloride and perchloroethylene as probably carcinogenic to humans (17, 18). Furthermore, several solvents including toluene, trichloroethylene, and perchloroethylene have possible endocrine disrupting properties and may interfere in the masculinization process in-utero (19). In a recent paper from the NORD-TEST study in Finland, Norway and Sweden (a registry-based case–control study of 8112 TGCT cases), maternal occupational exposure to aromatic hydrocarbon solvents including toluene showed a weak association with TGCT risk in their sons (20).
Exposure to heavy metals occurs primarily within the metal industry, including many occupational settings eg, in pigment and batteries production, metallurgy, and in welding. Several heavy metals are considered endocrine disruptors and have adverse effects on reproduction (21). For example, hexavalent chromium induces mitochondria-dependent apoptosis in male somatic cells and spermatogonial stem cells contributing to male reproductive abnormalities and infertility (22). IARC has classified chromium (VI) compounds, mixtures including nickel compounds, and nickel metal as carcinogenic to humans (mainly lung cancer) (23). Recently, also welding fumes were classified as lung carcinogen (24). A Canadian case–control study found an association between father’s employment in metal work and TGCT risk in their sons [odds ratio (OR) 3.28, 95% confidence interval (CI) 1.03–10.52, based on eight exposed case fathers and five control fathers] (25). The above-mentioned NORD-TEST study in Finland, Norway and Sweden showed an increased risk of TGCT in sons following paternal exposure to chromium in the category of high level/high probability of exposure (OR 1.37, 95% CI 1.05–1.79, based on 78 exposed case fathers and 200 control fathers) (26).
The present study investigates if parental occupational exposure to solvents and/or heavy metals is associated with a risk of TGCT in sons in NORD-TEST Denmark, complementing our earlier work in Finland, Norway, and Sweden (20, 26).
Methods
We conducted a nationwide registry-based case–control study on TGCT in Denmark. The reason for analyzing the data from Denmark separately from the other Nordic countries was the different source of occupational histories (20, 26). In the current study, information on occupation was retrieved from the Danish Supplementary Pension Fund (27), which keeps the entire individual employment history even after a person has retired or died, while in the earlier studies the occupational history was based on self-reported occupations in censuses carried out every 5–10 years in Finland, Norway and Sweden.
Study population
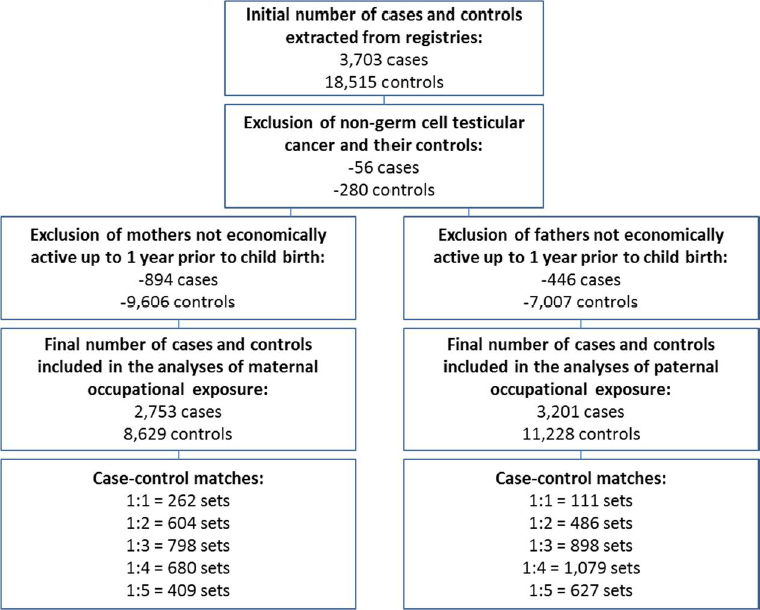
TGCT cases born in Denmark and diagnosed at age 14–49 years between 1981 and 2014 were identified via the population-based Danish Cancer Registry established in 1942. Reporting to the Danish Cancer Registry has been compulsory since 1987 and multiple notifications from different data sources has secured a high degree of completeness, eg, 89% of morphologically verified tumors (28). For each case, five controls born in Denmark were randomly selected from the Central Population Registry and matched for year of birth. Parents of cases and controls were identified from the Central Population Registry via their unique personal identity code, which is applied to all residents in Denmark (29).
In accordance with our previous NORD-TEST analyses of Finland, Norway and Sweden, we restricted the analyses to subjects with at least one parent having worked up to their child’s birth. We analyzed maternal and paternal occupational exposures separately, so the number of subjects included in each analysis differed as described in figure 1: 2753 cases and 8629 controls were included in analyses of maternal exposures and 3201 cases and 11 228 controls in analyses of paternal exposures.
Exposure assessment
Parental employment histories were obtained from the Danish Supplementary Pension Fund (ATP), including all employees from 16 years of age who have worked ≥9 hours per week in Denmark (27). Since 1964, for each employee, all jobs are recorded with information on company and start and end dates of employment. Statistics Denmark has classified companies into branch/industry codes (DSE77) based on a standard registration form filled out by the companies for tax purposes. Employers transfer information to each employee’s ATP account four times per year, and records are kept even when a company has closed or a person has emigrated or died (27). We used the Nordic Occupational Cancer Study job exposure matrix (NOCCA-JEM) for Denmark (NOCCA-DANJEM) to assign parental occupational exposures. The NOCCA-JEM were elaborated from the Finnish job exposure matric (FINJEM) and complementary data measurements by a team of selected Nordic exposure experts. The exposed jobs were those where a proportion of workers regularly experience occupational inhalatory exposure to a level exceeding the specified background level originating from non-occupational exposure. The NOCCA-DANJEM includes 24 chemical agents (ie, solvents, heavy metals, combustion products, animal- and wood dusts, asbestos, crystalline silica and formaldehyde) for the periods 1945–1959, 1960–1974, 1975–1984 and 1985–1994 (30). Mothers were considered exposed when holding an exposed job in the year of the index child’s birth, and fathers when holding an exposed job in the year before the index child’s birth. We chose these time windows of exposure because we were interested in the child’s exposure in utero as well as potential effects on the fathers’spermatozoids shortly before the child’s conception. We did not consider cumulative exposure because the sperm regeneration cycle only lasts 2.5-3 months. The NOCCA-JEM are linkable to the Nordic Classification of Occupations (NYK). Consequently, the exposure expert for NOCCA Denmark (JH) developed a crosswalk between NYK and DSE77 for the exposed NYK codes. We chose to assign exposures qualitatively, ie, unexposed or exposed, because some DSE77 codes corresponded to several NYK codes with different levels of exposures. When parents of study subjects had parallel jobs, they were assigned exposures from all jobs.
Statistical analysis
Pearson correlation coefficients were computed to assess pair-wise correlations between exposures.
We used conditional logistic regression analyses to estimate OR and 95% CI. Exposure to solvents and heavy metals were investigated individually and combined. First, three analyses were performed without any adjustment: “solvent only” with the reference group “non-exposed to any type of solvent”, “metals only” with the reference group “non-exposed to any type of heavy metal”, and “solvent and metals combined” with the reference group “non-exposed to both solvents and metals”. Second, we estimated OR for solvents (benzene, toluene, methylene chloride, perchloroethylene, 1,1,1-trichloroethane, trichloroethylene, and gasoline) adjusted for exposure to “any metal”, and for heavy metals (chromium VI, iron, lead, and nickel) adjusted for “any solvent”. Furthermore, we analyzed groups of solvents (aromatic hydrocarbon solvents and chlorinated hydrocarbon solvents). In an attempt to identify which specific solvent(s) or metal(s) have a potential independent association with TGCT risk, we created categories as follows: (i) no solvent or metal (reference category), (ii) x plus other solvent(s) or metal(s), and (iii) not x, but other solvent(s) or metal(s).
We considered father’s history of TGCT (identified from the cancer registry) and parental age at birth of the study subjects as potential confounders, meaning that if the OR changed >10%, the variable would be kept in the statistical models.
We did not retrieve data on urogenital malformations (cryptorchidism and hypospadias) for the current analyses because they were not confounders in the previous NORD-TEST analyses in Finland, Norway, and Sweden (7, 26).
Secondary analyses assessed TGCT risk by sub-type (seminomas and non-seminomas) and birth decade (1960s, 1970s, and 1980–90s) to examine whether potential associations varied by sub-type or over time. We assumed that exposure levels were higher in the past and envisaged the association would be stronger in the earlier decades if there was a true association (30). Wald test was used to test for homogeneity across sub-types and decades, and we considered P-values <0.05 to be statistically significant.
Analyses were carried out using SAS statistical package V.9.4 (SAS Institute, Inc, Cary, NC, USA) and Stata Statistical Software: release 14 (StataCorp, College Station LP, TX USA).
The Danish Data Protection Agency (J.nr 2013-41-1536) as well as the International Agency for Research on Cancer (IARC) ethics committee (project no. 12-10) have approved this study.
Results
In this analysis, we included 3421 TGCT cases and 14 024 controls.
Table 1 shows selected characteristics of the study population. Among the TGCT cases 49% were classified as seminomas and 51% as non-seminomas. Malignant teratoma was the most frequent tumour among the non-seminomas followed by embryonal carcinomas (including yolk sac tumor), and choriocarcinomas. The mean age at diagnosis of the cases was 29 years (median 29, range 14–49). The average parental age at childbirth was similar in cases and controls, and hence did not constitute a confounder in the analyses and was not included in the model.
Table 1
Characteristics of the study population of NORD-TEST Denmark.
Father’s history of testicular cancer was associated with TGCT in their sons (OR 1.88, 95% CI 1.57–2.26). However, it was not a confounder in our analyses because it was not associated with the selected parental occupational exposures and therefore not included in the final model.
Correlations between exposures to solvents & heavy metals
Pearson correlation coefficients between all exposures included in this analysis are shown in table 2. In mothers, exposures to any solvent and any heavy metal were very strongly correlated (r=0.80); 68% of exposed mothers were exposed to both solvents and metals, 22% to only solvents and 10% to only metals. In fathers, exposures to any solvent and any heavy metal were strongly correlated (r=0.74); 66% of exposed fathers had been exposed to solvents and metals, 11% to only solvents and 23% to only metals.
Table 2
Pairwise Pearson Correlation Coefficients (r) between exposures in mothers (italic font) and between exposures in fathers (bold font)
Parental occupational exposures to solvents & heavy metals
Table 3a shows that 9% of mothers of cases and of controls had been occupationally exposed to solvents and 8% had been exposed to metals in the year of their son’s birth. The adjusted OR for exposure to any solvent was 0.99 (95% CI 0.77–1.28), and for any metal 1.01 (95% CI 0.77–1.32). The OR associated with exposure to “at least one type of solvent and at least one type of heavy metal” was 1.05 (95% CI 0.88–1.24). We observed no statistically significant increased TGCT risk in relation to groups of or specific solvents or metals (table 3a; supplementary table S1, www.sjweh.fi/show_abstract.php?abstract_id=3732).
Table 3a
Associations of testicular germ cell tumor with maternal occupational exposure to solvents and metals. [OR=odds ratios; CI=confidence intervals]
| Exposures | Maternal exposure (N=11 382) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Control | % | Case | % | OR | 95% CI | OR a | 95% CI | |
| Solvents and/or heavy metals | ||||||||
| Neither solvent nor heavy metal | 7787 | 90 | 2487 | 90 | 1.00 | |||
| Any solvent or any heavy metal, but not both | 273 | 3 | 76 | 3 | 0.80 | 0.61–1.03 | ||
| Both solvent(s) and heavy metal(s) | 569 | 7 | 190 | 7 | 1.05 | 0.88–1.24 | ||
| Solvents | ||||||||
| No solvent | 7873 | 91 | 2507 | 91 | 1.00 | 1.00 | ||
| Any solvent | 756 | 9 | 246 | 9 | 1.00 | 0.86–1.16 | 0.99 | 0.77–1.28 |
| No solvent | 7873 | 91 | 2507 | 91 | 1.00 | 1.00 | ||
| Aromatic hydrocarbon and other solvents | 443 | 5 | 139 | 5 | 0.96 | 0.79–1.17 | 0.97 | 0.75–1.26 |
| Solvent(s) without aromatic hydrocarbon | 313 | 4 | 107 | 4 | 1.05 | 0.83–1.32 | 1.07 | 0.75–1.52 |
| No solvent | 7873 | 91 | 2507 | 91 | 1.00 | 1.00 | ||
| Chlorinated hydrocarbon and other solvents | 637 | 7 | 198 | 7 | 0.96 | 0.81–1.13 | 0.89 | 0.66–1.20 |
| Solvent(s) without chlorinated hydrocarbon | 119 | 1 | 48 | 2 | 1.21 | 0.86–1.70 | 1.18 | 0.83–1.67 |
| No solvent | 7873 | 91 | 2507 | 91 | 1.00 | 1.00 | ||
| Gasoline and other solvents | 108 | 1 | 34 | 1 | 1.03 | 0.69–1.52 | 1.02 | 0.63–1.65 |
| Solvent(s) without gasoline | 648 | 8 | 212 | 8 | 0.99 | 0.84–1.17 | 0.99 | 0.77–1.28 |
| Heavy metals | ||||||||
| No heavy metal | 7974 | 92 | 2543 | 92 | 1.00 | 1.00 | ||
| Any heavy metal | 655 | 8 | 210 | 8 | 1.00 | 0.85–1.18 | 1.01 | 0.77–1.32 |
| No heavy metal | 7974 | 92 | 2543 | 92 | 1.00 | 1.00 | ||
| Chromium and other heavy metal(s) | 207 | 2 | 65 | 2 | 1.02 | 0.76–1.36 | 1.02 | 0.71–1.47 |
| Metal(s) without chromium | 448 | 5 | 145 | 5 | 0.99 | 0.82–1.21 | 1.00 | 0.75–1.33 |
| No heavy metal | 7974 | 92 | 2543 | 92 | 1.00 | 1.00 | ||
| Iron and other heavy metal(s) | 147 | 2 | 49 | 2 | 1.03 | 0.74–1.44 | 1.04 | 0.70–1.53 |
| Metal(s) without iron | 508 | 6 | 161 | 6 | 0.99 | 0.82–1.19 | 1.00 | 0.75–1.33 |
| No heavy metal | 7974 | 92 | 2543 | 92 | 1.00 | 1.00 | ||
| Nickel and other heavy metal(s) | 176 | 2 | 58 | 2 | 1.03 | 0.76–1.40 | 1.04 | 0.73–1.48 |
| Metal(s) without nickel | 479 | 6 | 152 | 6 | 0.99 | 0.82–1.20 | 0.99 | 0.73–1.34 |
| No heavy metal | 7974 | 92 | 2543 | 92 | 1.00 | 1.00 | ||
| Lead and other heavy metal(s) | 617 | 7 | 198 | 7 | 0.99 | 0.84–1.17 | 1.00 | 0.76–1.31 |
| Metal(s) without lead | 38 | 0 | 12 | 0 | 1.19 | 0.62–2.31 | 1.20 | 0.60–2.43 |
Occupational exposures to solvents and heavy metals were more frequent in fathers than mothers (table 3b). About 19% of fathers of cases and of controls had been exposed to solvents and 22% to metals in the year before their son’s birth. The adjusted OR for exposures to any solvent and any metal were 1.04 (95% CI 0.90–1.21), and 0.99 (95% CI 0.86–1.14), respectively. The OR for “heavy metals other than lead” was 1.50 (95% CI 1.01–2.24), and most fathers in this category (N=144) were exposed to chromium (99%) and toluene (94%). The most frequent occupational titles in this category were “wooden and upholstered furniture factories”, “manufacture of building articles”, and “sawmills etc.”. None of the individual solvents showed any statistically significant association with TGCT in sons (table S1).
Table 3b
Associations of testicular germ cell tumor with paternal occupational exposure to solvents and metals. [OR=odds ratios; CI=confidence intervals]
| Exposures | Paternal exposure (N=14 429) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Control | % | Case | % | OR | 95% CI | OR a | 95% CI | |
| Solvents and/or heavy metals | ||||||||
| Neither solvent nor heavy metal | 8459 | 75 | 2408 | 75 | 1.00 | |||
| Any solvent or any heavy metal, but not both | 962 | 9 | 263 | 8 | 0.96 | 0.83–1.11 | ||
| Both solvent(s) and heavy metal(s) | 1807 | 16 | 530 | 17 | 1.04 | 0.94–1.16 | ||
| Solvents | ||||||||
| No solvent | 9110 | 81 | 2581 | 81 | 1.00 | 1.00 | ||
| Any solvent | 2118 | 19 | 620 | 19 | 1.04 | 0.94–1.14 | 1.04 | 0.90–1.21 |
| No solvent | 9110 | 81 | 2581 | 81 | 1.00 | 1.00 | ||
| Aromatic hydrocarbon and other solvents | 1565 | 14 | 456 | 14 | 1.02 | 0.91–1.14 | 1.02 | 0.88–1.20 |
| Solvent(s) without aromatic hydrocarbon | 553 | 5 | 164 | 5 | 1.09 | 0.91–1.31 | 1.11 | 0.89–1.38 |
| No solvent | 9110 | 81 | 2581 | 81 | 1.00 | 1.00 | ||
| Chlorinated hydrocarbon and other solvents | 1846 | 16 | 533 | 17 | 1.02 | 0.92–1.14 | 1.02 | 0.87–1.20 |
| Solvent(s) without chlorinated hydrocarbon | 272 | 2 | 87 | 3 | 1.12 | 0.88–1.44 | 1.12 | 0.87–1.45 |
| No solvent | 9110 | 81 | 2581 | 81 | 1.00 | 1.00 | ||
| Gasoline and other solvents | 813 | 7 | 222 | 7 | 0.96 | 0.82–1.12 | 0.95 | 0.77–1.17 |
| Solvent(s) without gasoline | 1305 | 12 | 398 | 12 | 1.08 | 0.96–1.22 | 1.08 | 0.92–1.26 |
| Heavy metals | ||||||||
| No heavy metal | 8770 | 78 | 2498 | 78 | 1.00 | 1.00 | ||
| Any heavy metal | 2458 | 22 | 703 | 22 | 1.02 | 0.93–1.12 | 0.99 | 0.86–1.14 |
| No heavy metal | 8770 | 78 | 2498 | 78 | 1.00 | 1.00 | ||
| Chromium and other heavy metal(s) | 1376 | 12 | 400 | 12 | 1.04 | 0.92–1.17 | 1.01 | 0.85–1.21 |
| Metal(s) without chromium | 1082 | 10 | 303 | 9 | 0.99 | 0.87–1.14 | 0.98 | 0.83–1.15 |
| No heavy metal | 8770 | 78 | 2498 | 78 | 1.00 | 1.00 | ||
| Iron and other heavy metal(s) | 1131 | 10 | 319 | 10 | 1.00 | 0.88–1.15 | 0.96 | 0.81–1.16 |
| Metal(s) without iron | 1327 | 12 | 384 | 12 | 1.04 | 0.92–1.17 | 1.01 | 0.86–1.17 |
| No heavy metal | 8770 | 78 | 2498 | 78 | 1.00 | 1.00 | ||
| Nickel and other heavy metal(s) | 1220 | 11 | 343 | 11 | 1.00 | 0.88–1.13 | 0.96 | 0.81–1.14 |
| Metal(s) without nickel | 1238 | 11 | 360 | 11 | 1.04 | 0.92–1.18 | 1.01 | 0.86–1.19 |
| No heavy metal | 8770 | 78 | 2498 | 78 | 1.00 | 1.00 | ||
| Lead and other heavy metal(s) | 2354 | 21 | 663 | 21 | 1.00 | 0.91–1.10 | 0.98 | 0.85–1.13 |
| Metal(s) without lead | 104 | 1 | 40 | 1 | 1.54 | 1.06–2.24 | 1.50 | 1.01–2.24 |
The associations of TGCT with maternal exposures to solvents or heavy metals showed no significant heterogeneity between seminomas and non-seminomas (table 4a). Paternal exposures also showed no marked heterogeneity by sub-type, with the exception of exposure to “gasoline” showing an OR of 1.20 (95% CI 0.91–1.59) for seminomas and an OR of 0.73 (95% CI 0.53–0.99) for non-seminomas (P=0.02) (table 4b).
Table 4a
The associations of testicular germ cell tumor with maternal occupational exposure to solvents and heavy metals by sub-type (non-seminoma and seminoma) [OR=odds ratios, CI=confidence intervals]
| Exposures | Maternal exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Non-seminoma (N=6031) | Seminoma (N=5351) | P-value b | |||||||
| Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | ||
| Solvents and/or heavy metals | |||||||||
| Neither solvent nor heavy metal | 4173 | 1303 | 1.00 | 3614 | 1184 | 1.00 | |||
| Any solvent or any heavy metal, but not both | 133 | 39 | 0.85 | 0.59–1.23 | 140 | 37 | 0.75 | 0.51–1.08 | 0.64 |
| Both solvent(s) and heavy metal(s) | 291 | 92 | 1.04 | 0.81–1.32 | 278 | 98 | 1.06 | 0.83–1.35 | 0.91 |
| Solvents | |||||||||
| No solvent c | 4217 | 1313 | 1.00 a | 3656 | 1194 | 1.00 a | |||
| Any solvent | 380 | 121 | 1.06 a | 0.74–1.51 | 376 | 125 | 0.93 a | 0.65–1.33 | 0.62 |
| Aromatic hydrocarbon and other solvents | 221 | 68 | 1.02 a | 0.71–1.48 | 222 | 71 | 0.93 a | 0.64–1.34 | 0.71 |
| Solvent(s) without aromatic hydrocarbon | 159 | 53 | 1.20 a | 0.72–1.99 | 154 | 54 | 0.96 a | 0.58–1.56 | |
| Chlorinated hydrocarbon and other solvents | 322 | 93 | 0.83 a | 0.54–1.28 | 315 | 105 | 0.95 a | 0.62–1.45 | 0.66 |
| Solvent(s) without chlorinated hydrocarbon | 58 | 28 | 1.48 a | 0.92–2.39 | 61 | 20 | 0.92 a | 0.54–1.54 | |
| Gasoline and other solvents | 52 | 17 | 1.14 a | 0.57–2.25 | 56 | 17 | 0.92 a | 0.47–1.82 | 0.68 |
| Solvent(s) without gasoline | 328 | 104 | 1.05 a | 0.74–1.51 | 320 | 108 | 0.94 a | 0.65–1.34 | |
| Heavy metals | |||||||||
| No heavy metal d | 4262 | 1332 | 1.00 a | 3712 | 1211 | 1.00 a | |||
| Any heavy metal | 335 | 102 | 0.94 a | 0.64–1.39 | 320 | 108 | 1.07 a | 0.74–1.56 | 0.63 |
| Chromium and other heavy metal(s) | 108 | 36 | 1.06 a | 0.65–1.74 | 99 | 29 | 0.97 a | 0.57–1.65 | 0.80 |
| Metal(s) without chromium | 227 | 66 | 0.88 a | 0.58–1.35 | 221 | 79 | 1.11 a | 0.75–1.65 | |
| Iron and other heavy metal(s) | 74 | 26 | 1.05 a | 0.61–1.79 | 73 | 23 | 1.02 a | 0.58–1.81 | 0.96 |
| Metal(s) without iron | 261 | 76 | 0.90 a | 0.60–1.37 | 247 | 85 | 1.09 a | 0.73–1.61 | |
| Nickel and other heavy metal(s) | 89 | 30 | 1.02 a | 0.62–1.66 | 87 | 28 | 1.06 a | 0.64–1.75 | 0.91 |
| Metal(s) without nickel | 246 | 72 | 0.90 a | 0.58–1.39 | 233 | 80 | 1.08 a | 0.71–1.63 | |
| Lead and other heavy metal(s) | 312 | 96 | 0.94 a | 0.63–1.39 | 305 | 102 | 1.06 a | 0.73–1.55 | 0.65 |
| Metal(s) without lead | 23 | 6 | 1.03 a | 0.39–2.70 | 15 | 6 | 1.44 a | 0.52–4.03 | |
Table 4b
The associations of testicular germ cell tumor with paternal occupational exposure to solvents and heavy metals by sub-type (non-seminoma and seminoma) [OR=odds ratios, CI=Confidence intervals]
| Exposures | Paternal exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Non-seminoma (N=7487) | Seminoma (N=6942) | P-value b | |||||||
|
|
|
||||||||
| Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | ||
| Solvents and/or heavy metals | |||||||||
| Neither solvent nor heavy metal | 4388 | 1218 | 1.00 | 4071 | 1190 | 1.00 | |||
| Any solvent or any heavy metal, but not both | 541 | 129 | 0.84 | 0.69–1.03 | 421 | 134 | 1.11 | 0.90–1.36 | 0.07 |
| Both solvent(s) and heavy metal(s) | 929 | 282 | 1.10 | 0.95–1.28 | 878 | 248 | 0.98 | 0.84–1.15 | 0.29 |
| Solvents | |||||||||
| No solvent c | 4766 | 1306 | 1.00 a | 4344 | 1275 | 1.00 a | |||
| Any solvent | 1092 | 323 | 1.10 a | 0.89–1.34 | 1026 | 297 | 0.99 a | 0.79–1.23 | 0.48 |
| Aromatic hydrocarbon and other solvents | 773 | 239 | 1.11 a | 0.90–1.38 | 792 | 217 | 0.94 a | 0.74–1.18 | 0.28 |
| Solvent(s) without aromatic hydrocarbon | 319 | 84 | 1.04 a | 0.77–1.40 | 234 | 80 | 1.19 a | 0.86–1.64 | |
| Chlorinated hydrocarbon and other solvents | 943 | 283 | 1.14 a | 0.91–1.42 | 903 | 250 | 0.89 a | 0.70–1.13 | 0.14 |
| Solvent(s) without chlorinated hydrocarbon | 149 | 40 | 0.96 a | 0.66–1.38 | 123 | 47 | 1.32 a | 0.92–1.88 | |
| Gasoline and other solvents | 396 | 127 | 1.20 a | 0.91–1.59 | 417 | 95 | 0.73 a | 0.53–0.99 | 0.02 |
| Solvent(s) without gasoline | 696 | 196 | 1.06 a | 0.85–1.32 | 609 | 202 | 1.09 a | 0.87–1.36 | |
| Heavy metals | |||||||||
| No heavy metal d | 4551 | 1259 | 1.00 a | 4219 | 1239 | 1.00 a | |||
| Any heavy metal | 1307 | 370 | 0.97 a | 0.80–1.18 | 1151 | 333 | 1.02 a | 0.82–1.25 | 0.76 |
| Chromium and other heavy metal(s) | 697 | 217 | 1.10 a | 0.86–1.40 | 679 | 183 | 0.93 a | 0.72–1.20 | 0.37 |
| Metal(s) without chromium | 610 | 153 | 0.90 a | 0.72–1.12 | 472 | 150 | 1.08 a | 0.86–1.37 | |
| Iron and other heavy metal(s) | 568 | 175 | 1.06 a | 0.82–1.35 | 563 | 144 | 0.88 a | 0.67–1.14 | 0.32 |
| Metal(s) without iron | 739 | 195 | 0.93 a | 0.75–1.15 | 588 | 189 | 1.11 a | 0.88–1.39 | |
| Nickel and other heavy metal(s) | 615 | 187 | 1.04 a | 0.82–1.31 | 605 | 156 | 0.89 a | 0.70–1.15 | 0.40 |
| Metal(s) without nickel | 692 | 183 | 0.92 a | 0.74–1.15 | 546 | 177 | 1.14 a | 0.89–1.44 | |
| Lead and other heavy metal(s) | 1248 | 348 | 0.96 a | 0.79–1.17 | 1106 | 315 | 1.00 a | 0.81–1.24 | 0.76 |
| Metal(s) without lead | 59 | 22 | 1.35 a | 0.79–2.31 | 45 | 18 | 1.71 a | 0.94–3.11 | |
OR by different birth decades were more variable (tables 5a and b). The associations between maternal occupational exposures to any solvent and TGCT risk in sons varied across decades (P=0.02), in particular for aromatic hydrocarbons (P=0.01). We observed a pattern where the OR for solvents were generally low in the 1960s, higher in the 1970s, and low in the 1980-90s with wide CI due to small numbers in each stratum. Additionally, OR for maternal exposure to all heavy metals varied across decades, although the patterns were different compared to those observed in solvents. The OR for exposure to specific heavy metals in mothers (chromium VI, iron, and nickel) were higher (>2.0) in the 1980-90s than in previous decades, with P-values for heterogeneity ≤0.02. The observed heterogeneity across decades for paternal occupational exposure to solvents as well as metals in relation to TGCT risk in sons was not significant (P=0.38 for any solvent and P=0.25 for any heavy metal).
Table 5a
The associations of testicular germ cell tumor with maternal occupational exposure to solvents and heavy metals by birth year. [OR=odds ratios, CI=confidence intervals]
| Maternal exposure | 1960–69 (N=2314) | 1970–79 (N=5831) | 1980–98 (N=3237) | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | ||
| Solvents and/or heavy metals | |||||||||||||
| Neither solvent nor metal | 1426 | 594 | 1.00 | 4008 | 1272 | 1.00 | 2353 | 621 | 1.00 | ||||
| Any solvent or any metal, but not both | 93 | 22 | 0.54 | 0.33–0.87 | 126 | 45 | 1.08 | 0.76–1.53 | 54 | 9 | 0.65 | 0.32–1.33 | 0.06 |
| Both any solvent and any metal | 128 | 51 | 0.98 | 0.70–1.37 | 290 | 90 | 0.99 | 0.77–1.27 | 151 | 49 | 1.26 | 0.89–1.76 | 0.50 |
| Solvents | |||||||||||||
| No solvent b | 1450 | 600 | 1.00 a | 4053 | 1281 | 1.00 a | 2370 | 626 | 1.00 a | ||||
| Any solvent | 197 | 67 | 0.70 a | 0.45–1.09 | 371 | 126 | 1.41 a | 0.99–2.01 | 188 | 53 | 0.67 a | 0.35–1.29 | 0.02 |
| Aromatic hydrocarbon and other solvents | 126 | 34 | 0.63 a | 0.40–1.00 | 217 | 78 | 1.44 a | 1.00–2.08 | 100 | 27 | 0.69 a | 0.35–1.36 | 0.01 |
| Solvent(s) without aromatic hydrocarbon | 71 | 33 | 1.17 a | 0.58–2.35 | 154 | 48 | 1.30 a | 0.79–2.14 | 88 | 26 | 0.65 a | 0.29–1.41 | |
| Chlorinated hydrocarbon and other solvents | 156 | 56 | 0.76 a | 0.43–1.35 | 318 | 96 | 1.14 a | 0.74–1.74 | 163 | 46 | 0.65 a | 0.33–1.30 | 0.25 |
| Solvent(s) without chlorinated hydrocarbon | 41 | 11 | 0.62 a | 0.32–1.22 | 53 | 30 | 1.92 a | 1.19–3.09 | 25 | 7 | 0.75 a | 0.29–1.97 | |
| Gasoline and other solvents | 19 | 8 | 0.83 a | 0.31–2.20 | 61 | 13 | 0.98 a | 0.48–2.02 | 28 | 13 | 1.09 a | 0.42–2.83 | 0.93 |
| Solvent(s) without gasoline | 178 | 59 | 0.70 a | 0.45–1.08 | 310 | 113 | 1.44 a | 1.01–2.04 | 160 | 40 | 0.64 a | 0.33–1.25 | |
| Heavy metals | |||||||||||||
| No heavy metal c | 1495 | 610 | 1.00 a | 4089 | 1308 | 1.00 a | 2390 | 625 | 1.00 a | ||||
| Any heavy metal | 152 | 57 | 1.25 a | 0.77–2.02 | 335 | 99 | 0.69 a | 0.47–1.02 | 168 | 54 | 1.78 a | 0.92–3.44 | 0.02 |
| Chromium and other heavy metal(s) | 33 | 13 | 1.23 a | 0.59–2.56 | 113 | 28 | 0.60 a | 0.35–1.00 | 61 | 24 | 2.35 a | 1.06–5.23 | 0.01 |
| Metal(s) without chromium | 119 | 44 | 1.26 a | 0.75–2.10 | 222 | 71 | 0.74 a | 0.49–1.11 | 107 | 30 | 1.58 a | 0.78–3.18 | |
| Iron and other heavy metal(s) | 29 | 12 | 1.23 a | 0.57–2.62 | 80 | 20 | 0.60 a | 0.34–1.07 | 38 | 17 | 2.47 a | 1.07–5.74 | 0.02 |
| Metal(s) without iron | 123 | 45 | 1.26 a | 0.75–2.09 | 255 | 79 | 0.72 a | 0.48–1.08 | 130 | 37 | 1.59 a | 0.80–3.18 | |
| Nickel and other heavy metal(s) | 32 | 13 | 1.19 a | 0.58–2.47 | 101 | 25 | 0.64 a | 0.38–1.07 | 43 | 20 | 2.28 a | 1.08–4.81 | 0.01 |
| Metal(s) without nickel | 120 | 44 | 1.27 a | 0.75–2.14 | 234 | 74 | 0.72 a | 0.47–1.11 | 125 | 34 | 1.45 a | 0.70–3.00 | |
| Lead and other heavy metal(s) | 152 | 56 | 1.23 a | 0.76–1.99 | 313 | 94 | 0.70 a | 0.47–1.03 | 152 | 48 | 1.75 a | 0.90–3.40 | 0.02 |
| Metal(s) without lead | 0 | 1 | 22 | 5 | 0.58 a | 0.21–1.65 | 16 | 6 | 2.26 a | 0.72–7.11 | |||
Table 5b
The associations of testicular germ cell tumor with paternal occupational exposure to solvents and heavy metals by birth year. [OR=odds ratios, CI=confidence intervals]
| Paternal exposure | 1960–69 (N=3884) | 1970–79 (N=7080) | 1980–98 (N=3465) | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | ||
| Solvents and/or heavy metals | |||||||||||||
| Neither solvent nor metal | 2254 | 720 | 1.00 | 4084 | 1155 | 1.00 | 2121 | 533 | 1.00 | ||||
| Any solvent or any metal, but not both | 251 | 65 | 0.80 | 0.60–1.07 | 485 | 144 | 1.05 | 0.86–1.29 | 226 | 54 | 0.96 | 0.70–1.31 | 0.33 |
| Both any solvent and any metal | 444 | 150 | 1.07 | 0.87–1.31 | 939 | 273 | 1.05 | 0.90–1.22 | 424 | 107 | 1.00 | 0.80–1.27 | 0.92 |
| Solvents | |||||||||||||
| No solvent b | 2406 | 755 | 1.00 a | 4421 | 1258 | 1.00 a | 2283 | 568 | 1.00 a | ||||
| Any solvent | 543 | 180 | 1.18 a | 0.89–1.56 | 1087 | 314 | 0.94 a | 0.77–1.16 | 488 | 126 | 1.14 a | 0.82–1.59 | 0.38 |
| Aromatic hydrocarbon and other solvents | 439 | 133 | 1.10 a | 0.82–1.47 | 813 | 239 | 0.95 a | 0.76–1.18 | 313 | 84 | 1.18 a | 0.82–1.69 | 0.53 |
| Solvent(s) without aromatic hydrocarbon | 104 | 47 | 1.74 a | 1.12–2.70 | 274 | 75 | 0.92 a | 0.67–1.25 | 175 | 42 | 1.08 a | 0.71–1.66 | |
| Chlorinated hydrocarbon and other solvents | 463 | 154 | 1.27 a | 0.90–1.80 | 956 | 273 | 0.91 a | 0.72–1.14 | 427 | 106 | 1.10 a | 0.79–1.55 | 0.24 |
| Solvent(s) without chlorinated hydrocarbon | 80 | 26 | 1.03 a | 0.65–1.62 | 131 | 41 | 1.08 a | 0.75–1.56 | 61 | 20 | 1.42 a | 0.80–2.52 | |
| Gasoline and other solvents | 212 | 64 | 1.10 a | 0.74–1.65 | 446 | 120 | 0.84 a | 0.63–1.11 | 155 | 38 | 1.08 a | 0.67–1.74 | 0.46 |
| Solvent(s) without gasoline | 331 | 116 | 1.20 a | 0.89–1.61 | 641 | 194 | 0.99 a | 0.79–1.23 | 333 | 88 | 1.16 a | 0.83–1.63 | |
| Heavy metals | |||||||||||||
| No heavy metal c | 2353 | 750 | 1.00 a | 4232 | 1196 | 1.00 a | 2185 | 552 | 1.00 a | ||||
| Any heavy metal | 596 | 185 | 0.87 a | 0.66–1.15 | 1276 | 376 | 1.11 a | 0.91–1.35 | 586 | 142 | 0.88 a | 0.64–1.20 | 0.25 |
| Chromium and other heavy metal(s) | 323 | 97 | 0.84 a | 0.60–1.18 | 724 | 216 | 1.14 a | 0.89–1.46 | 329 | 87 | 0.97 a | 0.66–1.43 | 0.35 |
| Metal(s) without chromium | 273 | 88 | 0.89 a | 0.65–1.22 | 552 | 160 | 1.09 a | 0.88–1.36 | 257 | 55 | 0.82 a | 0.58–1.17 | |
| Iron and other heavy metal(s) | 280 | 84 | 0.85 a | 0.60–1.20 | 605 | 172 | 1.06 a | 0.82–1.36 | 246 | 63 | 0.91 a | 0.61–1.36 | 0.57 |
| Metal(s) without iron | 316 | 101 | 0.89 a | 0.65–1.20 | 671 | 204 | 1.14 a | 0.92–1.41 | 340 | 79 | 0.86 a | 0.62–1.21 | |
| Nickel and other heavy metal(s) | 304 | 93 | 0.87 a | 0.63–1.21 | 650 | 185 | 1.06 a | 0.84–1.34 | 266 | 65 | 0.87 a | 0.59–1.27 | 0.53 |
| Metal(s) without nickel | 292 | 92 | 0.87 a | 0.63–1.20 | 626 | 191 | 1.15 a | 0.92–1.44 | 320 | 77 | 0.89 a | 0.63–1.26 | |
| Lead and other heavy metal(s) | 593 | 183 | 0.86 a | 0.65–1.14 | 1227 | 354 | 1.10 a | 0.90–1.34 | 534 | 126 | 0.87 a | 0.63–1.19 | 0.25 |
| Metal(s) without lead | 3 | 2 | 1.94 a | 0.32–11.69 | 49 | 22 | 2.00 a | 1.15–3.48 | 52 | 16 | 1.09 a | 0.57–2.08 | |
Discussion
We assessed the risk of GCNIS-related TGCT in sons in relation to mothers’ and fathers’ individual occupational exposure to solvents and heavy metals using data from a registry-based case–control study in Denmark. This analysis complements earlier work of our team studying the same parental exposures in relation to TGCT in their sons in Finland, Norway, and Sweden (20, 26), but with a different approach in the exposure assessment. In the analysis of Finland, Norway, and Sweden, the information on parental occupations before birth was based on census data (self-reported) that are updated every five or ten years whereas in the present analysis of Denmark it was based on ATP, which is continuously updated four times per year. While the job held during the year prior to childbirth is likely to be more accurate with the ATP data in Denmark, the categorization of jobs was somewhat different in Denmark compared to in Finland, Norway and Sweden, which potentially increased the exposure misclassification.
Overall, we found no association between parental exposure to solvents or heavy metals. There was only one exception where we observed an OR of 1.50 (95% CI 1.01–2.24) for fathers’ exposures to at least one of the heavy metals other than lead; the vast majority of those fathers were exposed to both chromium VI and toluene. The analyses by TGCT subtype (seminoma and non-seminoma) showed no major differences in the measures of association between exposures and TGCT in sons, and the analyses stratified by birth decades showed variable results without any clear patterns over time.
In a sensitivity analysis restricted to parents with census data from the year before childbirth, the previous NORD-TEST analysis from Finland, Norway, and Sweden showed an association between maternal occupational exposure to aromatic hydrocarbon solvents and TGCT risk in their sons (OR 1.53, 95% CI 1.08–2.17) (20). This result was not consistent with the overall results of the present study. However, in the stratified analyses by birth decade, we observed a similar OR of 1.44 (95% CI 1.00–2.08) for maternal exposure to aromatic hydrocarbon solvents in relation to TGCT risk in sons born in 1970–1979, during which we expected the exposure levels to be higher than in 1980–99. In contrast, there was a marginal inverse association between aromatic hydrocarbon and TGCT risk in sons born in 1960–1969 (OR 0.63, 95% CI 0.40–1.00) for which the reasons are unknown.
Another analysis of NORD-TEST in Finland, Norway, and Sweden showed an increased risk of TGCT in a group with higher mean levels and prevalence of paternal exposure to chromium (OR 1.37, 95% CI 1.05–1.79) (26). While the present study did not find an association between paternal exposure to chromium [ie, chromium and other metal(s)] and TGCT in sons, we found an increased risk of TGCT in the group where most of the fathers were exposed to both chromium and toluene (OR 1.50, 95% CI 1.01–2.24), and worked in wood related occupations. Such jobs include wood furniture factories, manufacture of building articles, and sawmills. A case-control study from Canada found an increased TGCT risk in sons whose fathers were wood processors (OR 10.5, 95% CI 1.2–91.1), metalworkers (OR 3.3, 95% CI 1.03–10.5), employees of metal products (OR 5.8, 95% CI 1.5–21.8) etc (25), which somewhat agrees with our results.
Wood preservatives contain chemicals to increase its durability and resistance for insects or fungus, examples include chromated arsenicals that contain copper and some combination of chromium and/or arsenic, pentachlorophenol (PCP), and creosote (31). We did not estimate exposure to these agents in our study, except chromium. Indeed, parental occupational exposure to chlorophenate fungicides has been associated with congenital anomalies of genital organs in the offspring (32). Pentachlorophenol and its bi-products are toxic, persistent and liable to bio accumulate in workers (33, 34).
Chance may also explain some of the associations observed in the present study. For example, maternal exposure to heavy metals showed elevated OR only in sons born in the 1980–90s, when occupational exposures supposedly were lower than during previous decades because of improved technologies and more stringent regulations etc (35).
Direct exposure to endocrine disrupting chemicals (EDC) has been suggested to contribute to the development of testicular dysgenesis syndrome (TDS) through interference with hormone synthesis, secretion and signaling (36). Experimental studies have suggested that prenatal exposure to EDC adversely affects the development of reproductive organs in offspring (10, 11) and supported a potential effect of solvent exposure, notably toluene, on the testes (37, 38). DNA damage in the male germline may lead to impaired growth and development in the offspring (39). Furthermore, concurrent exposure to heavy metals may increase genotoxic effects, including DNA repair inhibition (40). Although these mechanisms may explain the potential effect of paternal exposure to solvents and heavy metals on TGCT in the offspring, further studies are needed to understand the roles of solvent or heavy metal exposure, if any, in development of TGCT in sons.
The strengths of this study are the large study size, enrollment of all TGCT cases diagnosed in Denmark over more than three decades using a high quality national cancer registry, and the complete and objective reporting of the parents’ jobs.
Despite the strengths, our findings need to be interpreted while considering potential weaknesses of the study. Like any epidemiological study our study is probably subject to some exposure misclassification; applying a JEM to job or industry codes assumes that all workers in the same job have the same exposures even though substantial variability may exist (41). Furthermore, it was necessary to establish a crosswalk between the codes used in the NOCCA-DANJEM (NYK) and the DSE77 classification available in the ATP Denmark, which may have led to some additional exposure misclassification (42). On the other hand, the exposure assignment was done irrespective of disease status and in a standardized manner for all study participants, which can be an advantage compared to individual expert assessment (43). Another methodological challenge was that exposure to individual solvents and metals were moderately to strongly correlated (table 2). Consequently, it was not possible to create exclusive exposure categories for single exposures in any sensible way.
Concluding remarks
NORD-TEST Denmark does not provide strong evidence that parental occupational exposures to solvents or heavy metals are associated with testicular cancer risk in sons. We, however, recommend further studies on the potential associations of TGCT with fathers’ work in wood-related jobs and parental exposure to heavy metals and solvents. In particular, exposure to chromium and aromatic hydrocarbon solvents should be examined using toxicology approaches to identify possible mechanisms that may guide improvements in modeling individual exposures in future epidemiological studies.