Glyphosate [N-(phosphonomethyl)glycine] is a broad-spectrum herbicide that was first developed commercially for agricultural use in the early 1970s. Pesticides, including glyphosate, have been examined as potential risk factors for non-Hodgkin lymphoma (NHL) (1) and other lymphatic and hematopoietic cancers (2, 3). It has been hypothesized that pesticides may play a role in modifying immune function (4–6). Immune dysfunction is the most firmly established risk factor for NHL (7). However, currently, there is little evidence regarding this hypothesis for glyphosate specifically (5, 8).
In the 1980s and 1990s, population-based case-control studies were conducted in four states in the US Midwest and six Canadian provinces to examine putative associations between pesticide exposures, including glyphosate, and NHL. These studies comprise the North American Pooled Project (NAPP). Publication of individual study results showed suggestive associations between self-reported glyphosate use and NHL. In the Canadian study, the odds ratio (OR) for NHL was 1.26 [95% confidence interval (CI) 0.87–1.80] for the use of glyphosate (9). The OR was higher in a pooled logistic regression analysis of three case-control studies in Iowa/Minnesota, Kansas, and Nebraska (OR 2.1, 95% CI 1.1–4.0) (10). No analyses of specific NHL sub-types were conducted using data from any of these individual case-control studies.
A meta-analysis (1) that included these (9, 10) and other studies reported that glyphosate exposure was significantly associated with NHL overall (meta risk ratio (mRR) 1.5, 95% CI 1.1–2.0, N=6 papers). The risk of B-cell lymphoma was mRR 2.0, 95% CI 1.1–3.6 (11, 12).
In 2015, the International Agency for Research on Cancer (IARC) evaluated glyphosate carcinogenicity (8). This review resulted in the hazard classification of glyphosate as a “probable” (group 2A) human carcinogen based on limited evidence in humans for increased risk of NHL, sufficient evidence in experimental animals, and mechanisms that were pertinent to humans (8, 13). Mechanistic and other data supported the “probable” carcinogen conclusion by providing strong evidence for genotoxicity and oxidative stress, mechanisms of action that are relevant to humans (8).
The assessment of limited evidence from epidemiological studies was based on case–control studies in the U.S (10, 14), Canada (9), and Sweden (12, 15, 16) that reported increased associations with NHL that persisted after adjustment for use of other pesticides (8). However, in the Agricultural Health Study (AHS), no association was seen between glyphosate use and NHL overall in an initial publication (17). A recent evaluation from this cohort with additional follow-up and exposure information reported no association with NHL overall (RRhighest exposure quartile 0.87, 95% CI 0.64–1.20, P-trend=0.95) or any NHL sub-type (18).
The information available on the glyphosate and NHL association is somewhat limited. For example, only three studies (2, 9, 12) have reported on exposure metrics other than ever or never use. In addition, only three studies have reported any information on risk by NHL sub-type (11, 12, 18) which have different etiologies (19). Other limitations of previous studies include lack of adjustment for other pesticides. The goal of this pooled analysis was to provide a larger number of NHL cases and controls in order to allow more detailed analyses of possible relationships between NHL, specific NHL sub-types, and different metrics of glyphosate use.
Methods
Study population and exposure assessment
The NAPP involved pooling data from case–control studies of soft tissue sarcoma and lymphatic and hematopoietic cancers in the US and Canada. NHL cases were recruited from cancer registries and hospitals during the 1980s in four US states (Iowa/Minnesota, Kansas, and Nebraska) and between 1991 and 1994 in six Canadian provinces (Quebec, Ontario, Manitoba, Saskatchewan, Alberta, and British Columbia). Methods for each study have been previously described (9, 14, 20, 21). For the NAPP, the original histology codes used in each study were revisited to classify NHL cases using a single scheme [International Classification of Diseases for Oncology version 1 (ICD-O-1)].
Participants, or their proxies, provided information about demographic characteristics, pesticide use, agricultural exposures, and exposure to other known or suspected NHL risk factors, including lifestyle and medical and occupational history. Self-reported glyphosate use was examined using several exposure metrics: ever/never, duration (years used), frequency (days/year handled), and lifetime-days (number of years used multiplied by number of days/year handled). Categories were created for duration, frequency, and lifetime-days analyses based on the median of glyphosate used/handled among controls. Some participants had missing data for duration and frequency of glyphosate use despite reporting that they had ever used glyphosate. In duration and frequency analyses, values for missing data were assigned to cases and controls based on the median duration or frequency of reported glyphosate use among controls by state/province and 10-year age group (simple imputation) and were used for the main analyses. Ordinal analyses and associated trend tests were conducted to determine possible changes in association for increasing increments of every five years, five days/year, and ten lifetime-days of glyphosate use. Additional details on the original studies and on the methods in the pooled analysis are available in the supplementary material (www.sjweh.fi/show_abstract.php?abstract_id=3830, file 1).
Statistical analyses
Unconditional multiple logistic regression was performed using the LOGISTIC procedure of the SAS 9.4 statistical software package (SAS Institute, Cary, NC, USA) to calculate OR and 95% CI for associations between glyphosate exposure metrics (ever/never, duration, frequency, lifetime-days, and as ordinal variables) and associations with NHL overall and by histological sub-type [diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), small lymphocytic lymphoma (SLL), and other]. Complete methods for all statistical analyses are described in supplementary file 1. Primary logistic regression models (ORcrude) contained the following variables: age [age at diagnosis (cases); age at interview or death (controls)], state/province, sex, lymphatic or hematopoietic cancer in a first-degree relative (22, 23), response by a proxy (9, 14, 24), and use of any any personal protective equipment (PPE). Additional farming and medical factors considered as possible confounders were evaluated, but did not change the ORcrude by more than 10% and were not retained in finals models (supplementary file 1).
To evaluate whether the use of other pesticides might have confounded the association between specific pesticides, eg, glyphosate, and NHL, we used a two-pronged approach. First, a correlation matrix of pooled data was produced to determine the presence and extent of correlation between ever use of glyphosate and each individual herbicide, insecticide, and fungicide reportedly used by NAPP subjects. If the use of two pesticides are not correlated, then confounding cannot occur (25). Second, previously published articles based on the individual case–control studies comprising the NAPP were searched to identify any positive or significant relationships between individual pesticides and NHL risk. Pesticides that were most strongly correlated with glyphosate use (defined in this study as Spearman coefficients ≥0.35 and Cohen’s Kappa value ≥0.30) and that were statistically significantly or strongly associated with NHL in previous studies were evaluated as confounders. The herbicides 2,4-dichlorophenoxyacetic acid (2,4-D, r=0.35, P<0.001) (9, 14, 21) and dicamba (r=0.42, P<0.00019 (9, 10), as well as the insecticide malathion (r=0.38, P<0.0001) (9, 10), met both criteria and were therefore included in the more fully adjusted, secondary logistic regression models (ORadj).
Trends for duration, frequency, and lifetime-days of glyphosate use and NHL OR were deemed to be statistically significant if the two-sided P-value from the trend test (asymptotic Cochran-Armitage trend test) for glyphosate use was ≤0.05, or if the two-sided P-value for ordinal glyphosate use was ≤0.05. Subjects who never used glyphosate were the reference group for all analyses. There was a small proportion of subjects (N=175, 2.6% of all participants) with missing age values. These were imputed using simple imputation based on state/province- and case/control-specific means of age rounded to the nearest whole number. Sensitivity analyses were conducted by excluding proxy respondents from the main analyses.
Heterogeneity between the individual case–control studies comprising the NAPP was evaluated using the I2 statistic. The I2 statistic was calculated using study-specific OR for NHL overall in association with ever/never glyphosate use. Study-specific OR were generated from the NAPP data and not ascertained from previous publications of the individual case–control studies comprising the NAPP. The 95% CI for the I2 statistic were calculated since the number of studies was small (26, 27). Heterogeneity was determined to be statistically significant if the P-value for the I2 statistic was less than 0.05. STATA version 14.2 (StataCorp, College Station, TX, USA) was used to calculate the I2 statistic.
In the NAPP, statistically significant differences were evaluated using pairwise comparisons of the major histological sub-type OR in the SAS LOGISTIC procedure for ever/never glyphosate. Differences were determined to be statistically significant if the P-value for the Wald χ2 statistic was <0.05.
Both OR unadjusted and adjusted for other pesticides (ie, ORcrude and ORadj, respectively) were used in assessments of between-study heterogeneity and to determine potential differences in sub-type-specific odds ratios.
Ethics approval and consent to participate
Ethics approval for the pooled analysis was obtained from the University of Toronto Health Sciences Research Ethics Board (#25166) and an exemption was obtained from the US National Institutes of Health Office of Human Subjects Research (#11351). Investigators of individual studies received human subjects approval from their institutions for each study prior to collection of data.
Results
Characteristics of NHL cases and controls
A total of 1690 NHL cases and 5131 controls was available for analysis. All NHL cases and controls, including those with proxy respondents, were included in analyses of ever/never glyphosate use. For assessments involving duration of use, 1520 cases and 4183 controls were included. For frequency and lifetime-days analyses, 898 cases and 2938 controls were included. The numbers of cases and controls available for the sensitivity analysis excluding proxy respondents were smaller (figure 1). Characteristics of NHL cases and controls, including histological sub-types, are presented in Table 1.
Figure 1
Subjects in main and proxy respondent analyses of glyphosate use and NHL in the North American Pooled Project (NAPP). * Duration (years) information was not collected in Kansas, ** Frequency (days/year) information was not collected in Iowa, Minnesota, and Kansas.
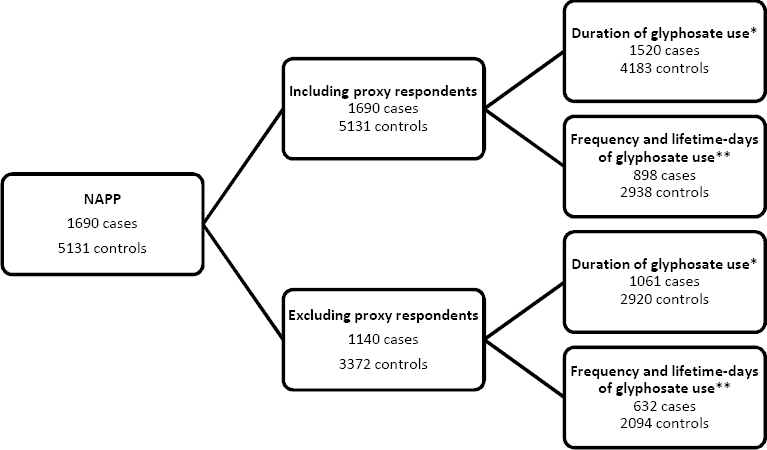
Table 1
Characteristics of non-Hodgkin lymphoma (NHL) cases and controls in the North American Pooled Project (NAPP). [OR=odds ratio; CI=confidence interval].
| Characteristics | Cases (N=1690) | Controls (N=5131) | OR a | 95% CI | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | % | N | % | |||
| Histological sub-type | ||||||
| Diffuse large B-cell lymphoma (DLBCL) | 647 | 38 | ||||
| Follicular lymphoma (FL) | 468 | 28 | ||||
| Small lymphocytic lymphoma (SLL) | 171 | 10 | ||||
| Other | 404 | 24 | ||||
| State/Province U.S | ||||||
| Nebraska | 385 | 22 | 1432 | 28 | ||
| Minnesota | 329 | 19 | 642 | 13 | ||
| Iowa | 293 | 17 | 603 | 12 | ||
| Kansas | 170 | 11 | 948 | 18 | ||
| Canada | ||||||
| Ontario | 142 | 8 | 585 | 11 | ||
| British Columbia | 126 | 7 | 230 | 4 | ||
| Quebec | 117 | 7 | 291 | 6 | ||
| Alberta | 65 | 4 | 196 | 4 | ||
| Manitoba | 34 | 2 | 113 | 2 | ||
| Saskatchewan | 29 | 2 | 91 | 2 | ||
| Age (years) b | ||||||
| ≥19–≤29 | 26 | 2 | 277 | 5 | ||
| ≥30–≤39 | 97 | 6 | 445 | 9 | ||
| ≥40–≤49 | 159 | 9 | 514 | 10 | ||
| ≥50–≤59 | 288 | 17 | 726 | 14 | ||
| ≥60–≤69 | 564 | 33 | 1264 | 25 | ||
| ≥70–≤79 | 402 | 24 | 1189 | 23 | ||
| ≥80–≤89 | 137 | 8 | 610 | 12 | ||
| ≥90 | 17 | 1 | 106 | 2 | ||
| Sex | ||||||
| Male | 1506 | 89 | 4424 | 86 | 1.00 | ref |
| Female | 184 | 11 | 707 | 14 | 0.94 | 0.75–1.17 |
| Respondent type | ||||||
| Self | 1140 | 67 | 3372 | 66 | 1.00 | ref |
| Proxy | 533 | 32 | 1692 | 33 | 1.03 | 0.90–1.17 |
| Unknown/missing | 17 | 1 | 67 | 1 | ||
| Lymphatic or hematopoietic cancer in a first-degree relative | ||||||
| No | 1493 | 88 | 4790 | 93 | 1.00 | ref |
| Yes | 139 | 8 | 202 | 4 | 2.13 | 1.69–2.67 |
| Unknown/missing | 58 | 3 | 139 | 3 | ||
| Ever diagnosed with selected medical conditions c | ||||||
| No | 1011 | 60 | 3346 | 65 | 1.00 | ref |
| Yes | 545 | 32 | 1389 | 27 | 1.12 | 0.92–1.37 |
| Unknown/missing | 134 | 8 | 396 | 8 | ||
| Ever used any type of personal protective equipment | ||||||
| No | 374 | 22 | 1127 | 22 | 1.00 | ref |
| Yes | 105 | 6 | 310 | 6 | 1.12 | 0.86–1.45 |
| Unknown/missing | 1211 | 72 | 3694 | 72 | ||
Glyphosate use and associations with NHL overall and by major histological sub-type
Overall, 113/1690 cases (7%) and 244/5131 (5%) controls reported having used glyphosate at any point in their lifetime. There was a significant association between ever use of glyphosate and NHL overall (ORcrude1.43, 95% CI 1.11–1.83) that was attenuated and no longer statistically significant when further adjusted for ever use of the pesticides 2,4-D, dicamba, and malathion (ORadj1.13, 95% CI 0.84–1.51) (Table 2). Significantly elevated OR found for DLBCL (ORcrude1.60, 95% CI 1.12–2.29) and other sub-types (ORcrude1.66, 95% CI 1.04–2.63) were not statistically significant after adjusting for other pesticides (DLBCL: ORadj1.23, 95% CI 0.81–1.88 and other sub-types: ORadj1.51, 95% CI 0.87–2.60). The near significant excess observed for SLL (ORcrude1.77, 95% CI 0.98–3.22), however, did not change appreciably after adjusting ORcrude for other pesticides (ORadj1.79, 95% CI 0.87–3.69). There was no association apparent with FL.
Table 2
Ever/never glyphosate use and associations with non-Hodgkin lymphoma (NHL) overall and histological sub-types in the North American Pooled Project [OR=odds ratio; CI=confidence interval]. Note: proxy respondents included.
| Never-used glyphosate | Ever-used glyphosate | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| N | ORa, b | N | OR a | 95% CI a | OR b | 95% CI b | |
| Controls | 4887 | 1.00 (ref) | 244 | ||||
| NHL overall | 1577 | 1.00 (ref) | 113 | 1.43 | 1.11–1.83 | 1.13 | 0.84–1.51 |
| Follicular lymphoma (FL) | 440 | 1.00 (ref) | 28 | 1.00 | 0.65–1.54 | 0.69 | 0.41–1.15 |
| Diffuse large B-cell lymphoma (DLBCL) | 602 | 1.00 (ref) | 45 | 1.60 | 1.12–2.29 | 1.23 | 0.81–1.88 |
| Small lymphocytic lymphoma (SLL) | 156 | 1.00 (ref) | 15 | 1.77 | 0.98–3.22 | 1.79 | 0.87–3.69 |
| Other | 379 | 1.00 (ref) | 25 | 1.66 | 1.04–2.63 | 1.51 | 0.87–2.60 |
When risks of NHL from glyphosate use were examined by duration, there was a general inverse trend except for SLL, for which the odds increased with longer duration (ORcrude1.49, 95% CI 0.63–3.58 for >0 and ≤3.5 years and ORcrude1.98, 95% CI 0.89–4.39 for >3.5 years, P-trend for ORcrude0.07) (Table 3). Results were similar for SLL when adjusted for other pesticides, but the trend was not statistically significant (P-trend for ORadj0.1).
Table 3
Duration of glyphosate use and associations with non-Hodgkin lymphoma (NHL) overall and histological sub-types in the North American Pool Project (NAPP). Note: data for glyphosate use not collected fo Kansas, proxy respondents included. [OR=odds ratio; CI=confidence interval.]
| Number of years of glyphosate use | N | OR a | 95% CI a | OR b | 95% CI b |
|---|---|---|---|---|---|
| Controls | |||||
| 0 | 3967 | ||||
| >0‒≤3.5 | 108 | ||||
| >3.5 | 108 | ||||
| P-trend c | |||||
| Ordinal (5 years) | 4183 | ||||
| P-value d | |||||
| NHL overall | |||||
| 0 | 1418 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 59 | 1.59 | 1.13–2.22 | 1.28 | 0.88–1.84 |
| >3.5 | 43 | 1.20 | 0.82–1.75 | 0.94 | 0.62–1.42 |
| P-trend c | 0.05 | 0.9 | |||
| Ordinal (5 years) | 1520 | 1.21 | 1.02–1.44 | 1.09 | 0.91–1.31 |
| P-value d | 0.03 | 0.3 | |||
| Follicular lymphoma (FL) | |||||
| 0 | 410 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 13 | 0.95 | 0.52–1.74 | 0.66 | 0.34–1.29 |
| >3.5 | 11 | 0.88 | 0.46–1.71 | 0.61 | 0.30–1.26 |
| P-trend c | 0.7 | 0.1 | |||
| Ordinal (5 years) | 434 | 0.99 | 0.71–1.39 | 0.86 | 0.59–1.26 |
| P-value d | 0.96 | 0.4 | |||
| Diffuse large B-cell lymphoma (DLBCL) | |||||
| 0 | 525 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 26 | 2.02 | 1.28–3.21 | 1.61 | 0.97–2.66 |
| >3.5 | 15 | 1.19 | 0.67–2.12 | 0.93 | 0.50–1.74 |
| P-trend c | 0.08 | 0.7 | |||
| Ordinal (5 years) | 566 | 1.28 | 1.03–1.60 | 1.16 | 0.91–1.48 |
| P-value d | 0.03 | 0.2 | |||
| Small lymphocytic lymphoma] (SLL) | |||||
| 0 | 144 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 6 | 1.49 | 0.63–3.58 | 1.43 | 0.55–3.72 |
| >3.5 | 8 | 1.98 | 0.89–4.39 | 1.94 | 0.79–4.80 |
| P-trend c | 0.07 | 0.1 | |||
| Ordinal (5 years) | 158 | 1.36 | 0.96–1.92 | 1.31 | 0.91–1.91 |
| P-value d | 0.08 | 0.2 | |||
| Other | |||||
| 0 | 339 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 14 | 2.08 | 1.14–3.78 | 1.82 | 0.95–3.50 |
| >3.5 | 9 | 1.32 | 0.64–2.71 | 1.14 | 0.51–2.51 |
| P-trend c | 0.1 | 0.4 | |||
| Ordinal (5 years) | 362 | 1.27 | 0.93–1.73 | 1.16 | 0.82–1.63 |
| P-value d | 0.1 | 0.4 | |||
a Adjusted for age, sex, state/province, lymphatic or hematopoietic cancer in a first-degree relative, use of a proxy respondent, use of any personal protective equipment.
b Adjusted for age, sex, state/province, lymphatic or hematopoietic cancer in a first-degree relative, use of a proxy respondent, use of any personal protective equipment, use of 2,4-D, use of dicamba, and use of malathion.
The OR from categorical analyses of frequency and lifetime-days use metrics showed mostly positive exposure-response gradients (Tables 4 and 5). Subjects who handled glyphosate for >2 days/year had NHL OR that were approximately twice that observed among participants who handled glyphosate for ≤2 days/year. These associations were significant both without and with adjustment for 2,4-D, dicamba, and malathion for NHL overall (ORcrude2.42, 95% CI 1.48–3.96; ORadj1.73, 95% CI 1.02–2.94) and for DLBCL (ORcrude2.83, 95% CI 1.48–5.41; ORadj2.14, 95% CI 1.07–4.28) (table 4). There were positive trends in associations for NHL overall and DLBCL with greater frequency of glyphosate use (P-trend for ORcrude0.002 and 0.01, respectively) (Table 4). In ordinal analyses, a greater number of days/year of glyphosate use was also positively associated with NHL overall (P-value for ORcrude0.02) and DLBCL (P-value for ORcrude0.04) (Table 4). However, these positive P-trend and P-values for NHL overall and DLBCL were no longer statistically significant when OR were further adjusted for 2,4-D, dicamba, and malathion (table 4). With respect to the lifetime-days analysis, positive exposure–response gradients were found in categorical and ordinal analyses of NHL overall, FL, DLBCL, and SLL, but only statistically significant for SLL with more lifetime-days of glyphosate use (P-value for ORadj0.03 in ordinal analyses) (table 5).
Table 4
Frequency of glyphosate handling and associations with non-Hodgkin lymphoma (NHL) overall and histological sub-types in the North American Pool Project (NAPP). Note: data for glyphosate use not collected fo Kansas, proxy respondents included. [OR=odds ratio; CI=confidence interval.]
| Number of days per year of glyphosate use | N | OR a | 95% CI a | OR b | 95% CI b |
|---|---|---|---|---|---|
| Controls | |||||
| 0 | 2789 | ||||
| >0‒≤2 | 106 | ||||
| >2 | 43 | ||||
| P-trend c | |||||
| Ordinal (5 days/year) | 2938 | ||||
| P-value d | |||||
| NHL overall | |||||
| 0 | 837 | 1.00 | ref | 1.00 | ref |
| >0‒≤2 | 31 | 1.03 | 0.67–1.60 | 0.74 | 0.46–1.19 |
| >2 | 30 | 2.42 | 1.48–3.96 | 1.73 | 1.02–2.94 |
| P-trend c | 0.002 | 0.2 | |||
| Ordinal (5 days/year) | 898 | 1.20 | 1.03–1.39 | 1.11 | 0.96–1.29 |
| P-value d | 0.02 | 0.2 | |||
| Follicular lymphoma (FL) | |||||
| 0 | 224 | 1.00 | ref | 1.00 | ref |
| >0‒≤2 | 7 | 0.81 | 0.35–1.84 | 0.47 | 0.19–1.15 |
| >2 | 8 | 2.21 | 0.99–4.93 | 1.33 | 0.55–3.23 |
| P-trend c | 0.2 | 0.9 | |||
| Ordinal (5 days/year) | 239 | 1.20 | 0.98–1.47 | 1.10 | 0.88–1.37 |
| P-value d | 0.07 | 0.4 | |||
| Diffuse large B-cell lymphoma (DLBCL) | |||||
| 0 | 342 | 1.00 | ref | 1.00 | ref |
| >0‒≤2 | 12 | 0.95 | 0.49–1.81 | 0.70 | 0.35–1.40 |
| >2 | 14 | 2.83 | 1.48–5.41 | 2.14 | 1.07–4.28 |
| P-trend c | 0.01 | 0.2 | |||
| Ordinal (5 days/year) | 368 | 1.20 | 1.01–1.42 | 1.14 | 0.96–1.35 |
| P-value d | 0.04 | 0.1 | |||
| Small lymphocytic lymphoma] (SLL) | |||||
| 0 | 66 | 1.00 | ref | 1.00 | ref |
| >0‒≤2 | 4 | 1.27 | 0.42–3.89 | 1.27 | 0.38–4.29 |
| >2 | 3 | 2.29 | 0.66–7.98 | 2.31 | 0.61–8.75 |
| P-trend c | 0.2 | 0.2 | |||
| Ordinal (5 days/year) | 73 | 1.20 | 0.90–1.59 | 1.18 | 0.87–1.59 |
| P-value d | 0.2 | 0.3 | |||
| Other | |||||
| 0 | 205 | 1.00 | ref | 1.00 | ref |
| >0‒≤2 | 8 | 1.49 | 0.66–3.32 | 1.14 | 0.49–2.65 |
| >2 | 5 | 2.26 | 0.85–5.99 | 1.61 | 0.58–4.50 |
| P-trend c | 0.07 | 0.4 | |||
| Ordinal (5 days/year) | 218 | 1.04 | 0.69–1.57 | 0.92 | 0.54–1.56 |
| P-value d | 0.9 | 0.8 | |||
a Adjusted for age, sex, state/province, lymphatic or hematopoietic cancer in a first-degree relative, use of a proxy respondent, use of any personal protective equipment.
b Adjusted for age, sex, state/province, lymphatic or hematopoietic cancer in a first-degree relative, use of a proxy respondent, use of any personal protective equipment, use of 2,4-D, use of dicamba, and use of malathion.
Table 5
Lifetime-days of glyphosate use and associations with non-Hodgkin lymphoma (NHL) overall and histological sub-types in the North American Pool Project (NAPP). Note: data for duration (years) of glyphosate use were not collected in Kansas. Data for frequency (days/year) of glyphosate handling were not collected in Kansas, Iowa, and Minnesota. Proxy respondents were included. [OR=odds ratio; CI=confidence interval.]
| Number of years used×days/year handled glyphosate | N | OR a | 95% CI a | OR b | 95% CI b |
|---|---|---|---|---|---|
| Controls | |||||
| 0 | 2793 | ||||
| >0‒≤3.5 | 76 | ||||
| >3.5 | 69 | ||||
| P-trend c | |||||
| Ordinal (10 lifetime-days) | 2938 | ||||
| P-value d | |||||
| NHL overall | |||||
| 0 | 841 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 25 | 1.20 | 0.74–1.95 | 0.87 | 0.52–1.45 |
| >3.5 | 32 | 1.55 | 0.99–2.44 | 1.08 | 0.66–1.77 |
| P-trend c | 0.05 | 0.9 | |||
| Ordinal (10 lifetime-days) | 898 | 1.06 | 1.01–1.11 | 1.04 | 1.00–1.08 |
| P-value d | 0.02 | 0.08 | |||
| Follicular lymphoma (FL) | |||||
| 0 | 225 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 6 | 1.03 | 0.43–2.48 | 0.64 | 0.25–1.62 |
| >3.5 | 8 | 1.33 | 0.60–2.94 | 0.75 | 0.31–1.80 |
| P-trend c | 0.5 | 0.4 | |||
| Ordinal (10 lifetime-days) | 239 | 1.07 | 1.01–1.12 | 1.05 | 1.00–1.11 |
| P-value d | 0.02 | 0.08 | |||
| Diffuse large B-cell lymphoma (DLBCL) | |||||
| 0 | 345 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 10 | 1.14 | 0.56–2.30 | 0.81 | 0.38–1.70 |
| >3.5 | 13 | 1.51 | 0.79–2.88 | 1.10 | 0.55–2.22 |
| P-trend c | 0.2 | 0.9 | |||
| Ordinal (10 lifetime-days) | 368 | 1.04 | 0.99–1.09 | 1.03 | 0.98–1.07 |
| P-value d | 0.1 | 0.2 | |||
| Small lymphocytic lymphoma] (SLL) | |||||
| 0 | 66 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 2 | 1.04 | 0.24–4.58 | 1.03 | 0.22–4.84 |
| >3.5 | 5 | 2.13 | 0.76–5.96 | 2.19 | 0.70–6.86 |
| P-trend c | 0.2 | 0.2 | |||
| Ordinal (10 lifetime-days) | 73 | 1.09 | 1.02–1.16 | 1.08 | 1.01–1.16 |
| P-value d | 0.01 | 0.03 | |||
| Other | |||||
| 0 | 205 | 1.00 | ref | 1.00 | ref |
| >0‒≤3.5 | 7 | 1.93 | 0.82–4.51 | 1.42 | 0.59–3.46 |
| >3.5 | 6 | 1.69 | 0.68–4.15 | 1.28 | 0.49–3.34 |
| P-trend c | 0.1 | 0.5 | |||
| Ordinal (10 lifetime-days) | 218 | 1.04 | 0.96–1.13 | 1.02 | 0.93–1.12 |
| P-value d | 0.3 | 0.7 | |||
a Adjusted for age, sex, state/province, lymphatic or hematopoietic cancer in a first-degree relative, use of a proxy respondent, use of any personal protective equipment.
b Adjusted for age, sex, state/province, lymphatic or hematopoietic cancer in a first-degree relative, use of a proxy respondent, use of any personal protective equipment, use of 2,4-D, use of dicamba, use of malathion.
Adjusting for other pesticide usage resulted in the attenuation of P-trend and P-values for nearly all glyphosate use metrics for all sub-types except for SLL (tables 3–5). There were statistically significant trends for OR of NHL overall from categorical analyses of number of years (P-trend for ORcrude0.05) (Table 3), number of days per year (P-trend for ORcrude0.002) (Table 4), and number of lifetime-days (P-trend for ORcrude0.05) (Table 5) of glyphosate use. However, trends were diminished and no longer statistically significant when OR for NHL overall were adjusted for the use of 2,4-D, dicamba, and malathion (P-trends for ORadj0.9, 0.2, and 0.9 for number of years, days per year, and lifetime-days, respectively) (tables 3, 4, and 5).
Sensitivity analyses excluding proxy respondents
A sensitivity analysis was performed by excluding cases and controls whose data were provided by proxy respondents (supplementary file 2, table S1) and results were compared with the main analysis (tables 2–5). The overall pattern of OR estimates and trends were similar in sensitivity and main analyses. As with the main analyses, adjustment for use of 2,4-D, dicamba and malathion tended to reduce OR and weaken trends for all metrics of glyphosate use. For SLL, trends of increasing OR for SLL in association with longer duration, greater frequency and lifetime-days of categorical glyphosate use were marginally stronger compared to main analyses. A full description of sensitivity analysis results is in supplementary file 2.
Between-study heterogeneity and differences in odds ratios between NHL major histological sub-types
There was no apparent heterogeneity between the case–control studies comprising the NAPP based on the analyses of ever/never glyphosate use and OR for NHL overall. When OR for ever/never glyphosate use were adjusted for other pesticide uses (ie, ORadj), there was a statistically significant difference observed between FL and SLL (P=0.04) and borderline statistically significant differences between FL and other sub-types (P=0.05) and FL and DLBCL (P=0.08). These heterogeneity test results supported pooling across the NAPP studies, but also indicated that differences exist that may make it worthwhile to consider the histological sub-types individually. See supplementary file 2 for complete results.
Discussion
The objective of this study was to evaluate potential associations between glyphosate use and NHL in the NAPP, a pooled dataset that allowed for a more comprehensive analysis than previously possible in individual studies. Results from this analysis provide some limited, but inconsistent, evidence for an association between NHL overall and ever reported use of glyphosate. Although there was a statistically significant association between ever glyphosate use and NHL overall, this association was attenuated and became no longer statistically significant when adjusted for reported use of 2,4-D, dicamba, and malathion. However, the significant excess risk among those who reported use of glyphosate for ≥2 days per year was not eliminated by adjustment for other pesticides, although the trend test was not statistically significant after adjustment for other pesticides. There was no pattern of increasing risk of NHL overall with increasing years of use of glyphosate. Finally, there was a small excess of NHL overall with lifetime-days as an ordinal metric that remained of borderline statistical significance after adjustment for other pesticides and after restricting analyses to those without proxy respondents.
In analyses of NHL sub-types, the most consistent evidence was found for SLL, where positive patterns were observed for duration, frequency, and lifetime-days of glyphosate use. However, OR, P-trend, and P-values in sub-type analyses were typically not statistically significant. In sub-type analyses adjusted for use of other pesticides and excluding responses from proxies, there was a significant association between the ordinal metric for lifetime-days of glyphosate use and SLL. Pairwise comparisons of the NHL sub-type analyses demonstrated that OR for FL and SLL were significantly different from each other in the ever versus never glyphosate use analysis. Results for DLBCL, SLL, and other sub-types looked somewhat different than that for FL in that they tended to have slightly elevated OR for ever glyphosate use and for >3.5 lifetime-days of glyphosate use. FL mostly had deficits.
The ORadj for NHL overall in association with ever use of glyphosate in the current analysis of the NAPP differed from OR reported in earlier individual analyses (9) (10). These differences may be due to the selection of the sample and statistical methods chosen to model NHL OR. For example, previous publications included only men (9, 10), while this analysis included both men and women. We also included a different set of covariates than did the original studies, using criteria described in the Methods section, and we did not exclude subjects with missing pesticide use data.
The contributing studies and our pooling activities have some methodological limitations. Proxy respondents provided information for about one third of the cases and controls. Because proxies cannot provide as much detail as the farmer regarding occupational exposures on a farm (28), exposure misclassification from proxies might bias estimates of relative risk. Accordingly, we performed analyses with proxies excluded to evaluate the potential for such bias and found that the pattern of results from these analyses were generally similar to those with proxies included.
Although farmers, who provided much of the information on pesticide use in this pooled study, can provide reliable information on pesticide use (29), some exposure misclassification was likely to have occurred in all studies because participants were asked to recall pesticide use for a number of years in the past. Non-differential pesticide exposure misclassification would tend to bias estimates of relative risk toward the null (30, 31). The case–control studies included in our pooling project, however, may also experience differential exposure misclassification, which can bias risk estimates toward or away from the null. There was some information available to evaluate misclassification of pesticide exposure in some of the source studies. In the study in Kansas, pesticide suppliers provided information on crops and pesticide purchases for a sample of 130 subjects with farming experience, and the data from farmers were found to be reasonably accurate (20). In the Nebraska study, case recall bias was assessed by comparing information on pesticide use that was volunteered versus information that required probing by the interviewer (21, 32), and little evidence for case recall bias was found. In Canada, a validation pilot study on a sample of reported pesticide usage was reviewed with purchases from pesticide supply companies, with a high degree of concordance between the two sources (9). There was a moderate level of correspondence between pesticide use information reported by farmers and their pesticide suppliers in Kansas (20, 32). In Nebraska, the number of insecticides and herbicides voluntarily identified by subjects and their surrogates was similar and suggested the absence of case-response bias, but probing increased the number of positive responses for individual agents (33).
Adjusting for 2,4-D, dicamba, and malathion was used to attempt to disentangle the effect of glyphosate on NHL from other pesticides. Some studies have suggested that these chemicals may be independently associated with NHL (9, 10, 14). This approach indicated that some confounding may occur without such adjustments. Unmeasured confounding by other pesticides and agricultural exposures cannot be completely ruled out, but we evaluated and adjusted for a number of factors noted as potential confounders in the literature and/or specifically in these data. Thus, any such confounding would have to be from a new and completely unsuspected risk factor for NHL and this unknown risk factor would also need to be associated with glyphosate use.
The strengths of this analysis are the large numbers of exposed cases and controls that resulted in more precise results than possible in previous smaller studies with lower power, information on NHL sub-types, detailed information on use of glyphosate and other pesticides, and availability of information on many potential NHL risk factors. Both agricultural and non-agricultural uses of glyphosate were reported by cases and controls, making this evaluation broadly relevant to a wide range of glyphosate use scenarios. While results are not independent from previous reports of individual studies included in this pooling (9, 14, 20, 21), evaluations by histological sub-type and the use of informative glyphosate use metrics are new.
NHL is a constellation of heterogeneous cancers with different biological properties that may have distinct etiologies (34). The large size of the NAPP made it possible for the first time to assess whether analyses of sub-types would reveal etiological heterogeneity. However, it is important to note that the classification of NHL sub-types has changed over time. Since we relied on previously collected data, we were not able to use the most current classification scheme (SEER Lymphoma Recode), but rather relied on classification in effect at the time of data collection. For example, in the most recent classification scheme, SLL and chronic lymphocytic leukemia (CLL) are classified together as they have similar etiologies, while our analysis only includes SLL. In a publication from the AHS (18), there was no association between glyphosate use and the most recent classification scheme that includes both SLL and CLL.
In conclusion, this analysis of pooled data from the NAPP provides some limited evidence of an association between glyphosate and NHL. The association between glyphosate and NHL overall appears to be confounded by exposure to other pesticides. However, increased OR for NHL overall with greater days/year and lifetime-days of glyphosate use were not entirely eliminated by adjustment for other pesticides. In subtype analyses, although based on a relatively small number of cases, SLL showed the most consistent association and exposure–response pattern with the different glyphosate use metrics and with control for other pesticides. There were some elevated OR for DLBCL and other sub-types, but these were not consistent across the different glyphosate use metrics. Larger numbers of NHL cases who used glyphosate would be required to further identify potential risks by NHL sub-type.



