Parkinson’s disease (PD) is a debilitating chronic neurodegenerative disease, which affects nearly five million individuals aged >50 years worldwide – a number which is projected to double by 2030 (1). Working rotating night shifts, a common feature of the western world, disrupts a range of normal physiological functions that follow a circadian rhythm, including the secretion of melatonin (2, 3), and has wide impacts on health (4). Previous epidemiological studies suggest that working rotating night shifts is associated with a higher risk of cancer (5) and a lower risk of PD (6).
Oxidative stress has been implicated in PD pathogenesis (7, 8); however, whether the potent antioxidant melatonin is beneficial in the prevention and treatment of PD remains unclear (9). Mitochondrial dysfunction is also considered as a pathogenic mechanism for PD, and melatonin is selectively taken up by mitochondria, which is not the case for many other antioxidants (9, 10). While melatonin has been hypothesized to be protective in PD pathogenesis primarily because of its antioxidant properties (11–16), animal models of PD using melatonin treatment in pharmacological doses have not been entirely consistent (17–19). For example, one study examined the potential effect of light in rat models of parkinsonism, showing that exposure to light significantly reduced the severity of PD symptoms while exogenous melatonin deteriorated the symptoms (19). The relevance of these animal findings for humans remains uncertain.
To our knowledge, the only previous observational study that examined the association between night work and PD risk is a prospective cohort study of nurses with 181 incident PD cases among 84 794 female nurses (7). This study found that women who worked rotating night shifts for ≥15 years had a 50% lower risk of PD [95% confidence interval (95% CI) 0.26–0.97, P for trend=0.01].
To follow-up these earlier results, we conducted a case–control study, including 1807 patients with idiopathic Parkinson’s disease to examine whether a history of night shift work is associated with Parkinson’s disease. We hypothesized that lowered nightly melatonin secretion and circadian disruption, as encountered by persons who work night shift, reduces PD risk.
Methods
Patients
Between January 2008 and December 2010, we established one of the largest case–control studies of PD worldwide by taking advantage of the rich Danish population registers. For initial case selection, we applied a set of inclusion criteria to the files of the Danish Hospital Register for the period 1996–2009. Specifically, eligible participants had to have had a primary hospital discharge diagnosis of PD (ICD-8 code 342 and ICD-10 code G20) at one of ten neurological treatment centers in Denmark; be alive at the date of interview, which took place between January 2008 and December 2010; speak Danish or English; be well enough to participate; and be ≥35 years. To ensure appropriate survival, patients had to be <70 years old if diagnosed between 1996–2001, and <80 years old if diagnosed between 2002–2009. Applying these criteria, we identified a total 2762 eligible patients; of these, we excluded 179 (6.5%) participants because a screening of their medical records before the interview precluded a diagnosis of idiopathic PD or because they themselves, at the interview, denied having PD. Of the remaining 2583 patients, 2086 (80.8%) agreed to a full interview, and we obtained medical records for 2066 (99.0%) of them.
Under the supervision of a movement disorder specialist, trained reviewers blinded to the exposure status applied standard diagnostic criteria of the United Kingdom Brain Bank (20) and the Gelb criteria (21) to all 2066 medial records. Our goal was to identify and retain only idiopathic PD cases and eliminate cases with atypical features or other disorders. The varying degree of completeness of medical records required some flexibility in their evaluation, but in general we considered a PD case to be idiopathic if (i) ≥2 of 4 cardinal symptoms (resting tremor, bradykinesia, rigidity, asymmetrical onset) were present; and the individual (ii) responded to anti-parkinsonian medication; (3) had no atypical features (ie, dementia before development of cardinal symptoms, early falls, severe symptomatic dysautonomia, rapid deterioration within two years of disease onset, sudden onset of symptoms, supranuclear gaze palsy, hallucinations unrelated to medication, freezing phenomena, Babinsky sign); and (4) displayed no signs of a differential diagnosis, eg, cerebrovascular disease. We used the first report of any of the four cardinal symptoms (resting tremor, bradykinesia, rigidity, asymmetrical onset) in the medical records, or first date of use of PD medication, or first date of physician PD diagnosis (self reported), whichever came first, as the date of diagnosis of PD in our analyses. Applying these criteria during medical record review, we concluded that 1828 (88.5%) of the 2066 interviewed patients indeed had idiopathic PD.
Population controls
For each of the 2583 patients that were initially contacted for interview, we randomly picked 5–10 controls from the Danish Central Population Register. Eligible controls were matched to PD cases by year of birth and gender and had to be alive and without a prior hospital diagnosis of PD at index date (ie, the date of diagnosis of their respective case). We contacted the 5 selected controls by phone in random order until the first consented to participate. Of a total of 3626 controls contacted, 1909 (52.6%) consented to participate and completed an interview.
Structured telephone interviews
We obtained study subjects’ current addresses and vital status from the Central Person Registry and mailed invitation letters to all potential participants. After having obtained written informed consent, trained interviewers contacted each respective participant by telephone to conduct a structured interview. Individuals with speaking difficulties were provided with the opportunity to respond to a mailed paper questionnaire instead of the telephone interview (N=619), and the proportion of cases and controls who returned the completed questionnaire was roughly comparable (11% versus 9%). On average, interviews of PD cases took place 5–6 years after their date of diagnosis (median, 5.4 years, 25–75th percentile, range 3.1–9.0 years).
Exposure assessment
During the interview, we elicited information on occupational history. Specifically, we asked for each job a study subject had held for over one year throughout their entire working career: “Did you work between 19:00–09:00 hours within this job?” If yes, we further queried whether this work took place “mainly during the evening (15:00–24:00 hours)”, “mainly during the night (24:00–09:00 hours)”, or whether they switched between evening and night shifts. We classified night work history into the following primary categories: day work only (ie, never evening or night shift work); ever worked primarily evening work and never night work; ever worked primarily rotating day, evening and night shift work; ever worked permanent night work; and ever worked any type of overnight work (either rotating or permanent).
Other covariates
Information on several important lifestyle covariates was also elicited during the standardized telephone interview. For example, we queried detailed smoking history, caffeine intake, and alcohol consumption. In addition, we queried each participant’s educational background, including their highest attained education, and family history of PD, which was defined as having a first-degree (ie, parent or sibling) relative with the disease. Urbanization was defined based on a participants’ home municipality at the index date from the Central Population Register.
Statistical analysis
Among the 1828 patients with idiopathic PD and their 1909 matched controls, we employed additional exclusion criteria aimed at eliminating individuals with neurological conditions potentially unrelated to idiopathic PD. Specifically, we excluded 14 patients and 22 controls who had had a hospital contact for dementia (ICD-8 codes 290.09-290.19 or 293.09; ICD-10 codes F00-03, F05.1 or G30) or cerebrovascular disease (ICD-8 codes 430-438; ICD-10 codes I60-69, G45 or G46) during any time between the start of the Hospital Register in 1977 and up to three years before the index date. According to medical records, one PD case appeared to have experienced their first symptom onset only after the date of the interview and was therefore excluded. Lastly, we excluded 34 cases and 35 controls without a work history (ie, they were permanently out of the workforce). After all exclusions, 1779 cases and 1852 controls remained to form the base population for our analyses.
To evaluate associations between night shift work and PD, we calculated odds ratios (OR) and 95% CI using conditional and unconditional logistic regression models. Because results from both models were virtually identical, and because we used unconditional logistic regression models in stratified analyses, we chose to present these as our primary models throughout the manuscript. Individuals who reported never having worked any other schedules besides day work represented the reference group in all analyses. As described earlier, we categorized our exposure (ie, shift work) in a number of different ways. Individuals who had ever held a job with primarily evening and day work constituted another exposure category, as did those who had ever held a job with primarily rotating day, evening, and night shift work. Lastly, we formed a separate group of individuals who had ever worked permanent night work in any of the jobs they held. In some instances, we collapsed rotating and permanent night work into one category. Given our classification of shift work history, a person could contribute to multiple categories. Further, we assessed lifetime duration in each of these non-day job types (<10, 10–19, ≥20 years).
In our primary analyses, we adjusted for year of birth (continuous) and sex. Multivariate analyses were additionally adjusted for age at index date (continuous), smoking history in pack-years (0, <10 years, 10–20 years, 20–30 years, 30–40 years, >40 years and “missing”), caffeine intake (cups/week; continuous), alcohol consumption (drinks/week; continuous), and degree of urbanization [capital (Copenhagen), provincial town, rural area, peripheral region]. In secondary analyses we also adjusted for family history of PD (none, suspected, diagnosed) and highest attained education (basic: 7–12 years; vocational: 10–12 years; higher: ≥13 years), but because results remained virtually unchanged they were not retained in the final models. We stratified by gender and smoking status, and calculated P-values for trend, using midpoints for each duration category and reporting the Wald statistic. To evaluate the possibility for selection bias, we implemented a 15-year lag time and restricted the secondary analyses to cases (and their controls) diagnosed in 2002 and later.
We used STATA version 11 (Stata Corp, College Station, TX, USA) for all analyses. The Ethical Committee and the Danish Data Protection Agency as well as UCLA and the Brigham and Women’s Hospital approved this study. All study subjects provided written consent to participate in this study.
Results
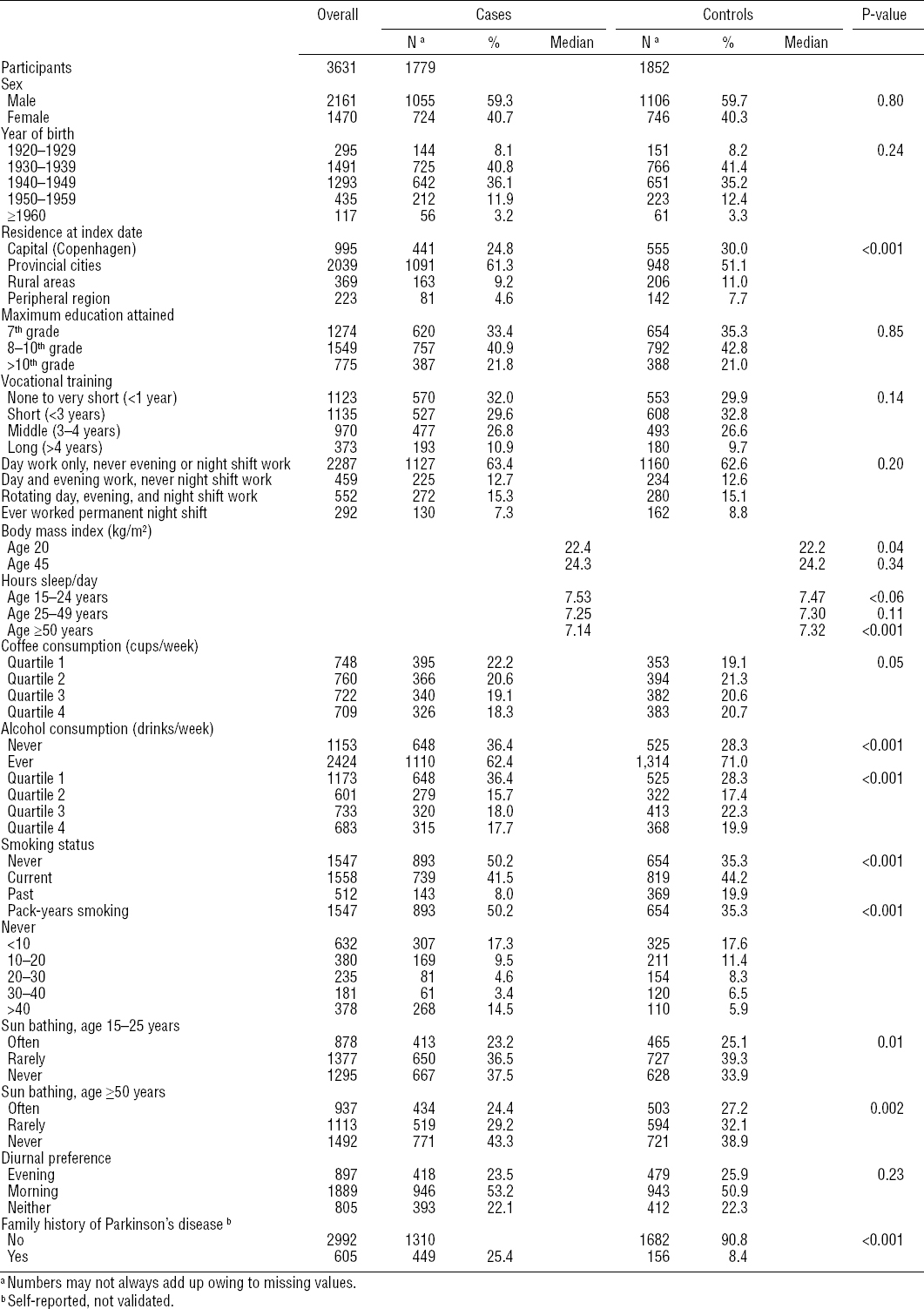
Table 1 summarizes lifestyle characteristics and shift work history of the 1779 cases and 1852 controls who participated in this study. Overall, cases tended to be less likely to consume caffeine or alcohol, take a sun bath (as a surrogate for vitamin D exposure), or smoke (although if they smoked, they reported more pack-years), and more men than women developed PD. They also appeared to have fewer hours of sleep per day.
Our first analyses examined whether experience of any type of atypical work schedule (day and evening, all schedules including night shifts, permanent night shifts) affected the risk of PD. Our next analyses were conducted to examine the effects of shift work duration.
Combining men and women, overall, we found no association between shift work schedules and PD risk. Danes who reported ever having worked overnight shifts (ie, either rotating or permanent night shifts) experienced similar PD risk (age-adjusted OR 0.96, 95% CI 0.81–1.12), as those who reported never having worked an evening or night shift. Additional adjustment for smoking, coffee and alcohol consumption, as well as urbanization did not substantially alter this risk estimate (OR 1.01, 95% CI 0.86–1.21). Risks were similar for both men and women separately (table 2). To evaluate the potential for selection bias, we excluded cases that were diagnosed before 2002 and conducted 15-year lag time analyses; reassuringly, results remained essentially unchanged.
Table 2
Odds ratios (OR) and 95% confidence intervals (95% CI) for Parkinson’s disease by types of night shift-work.
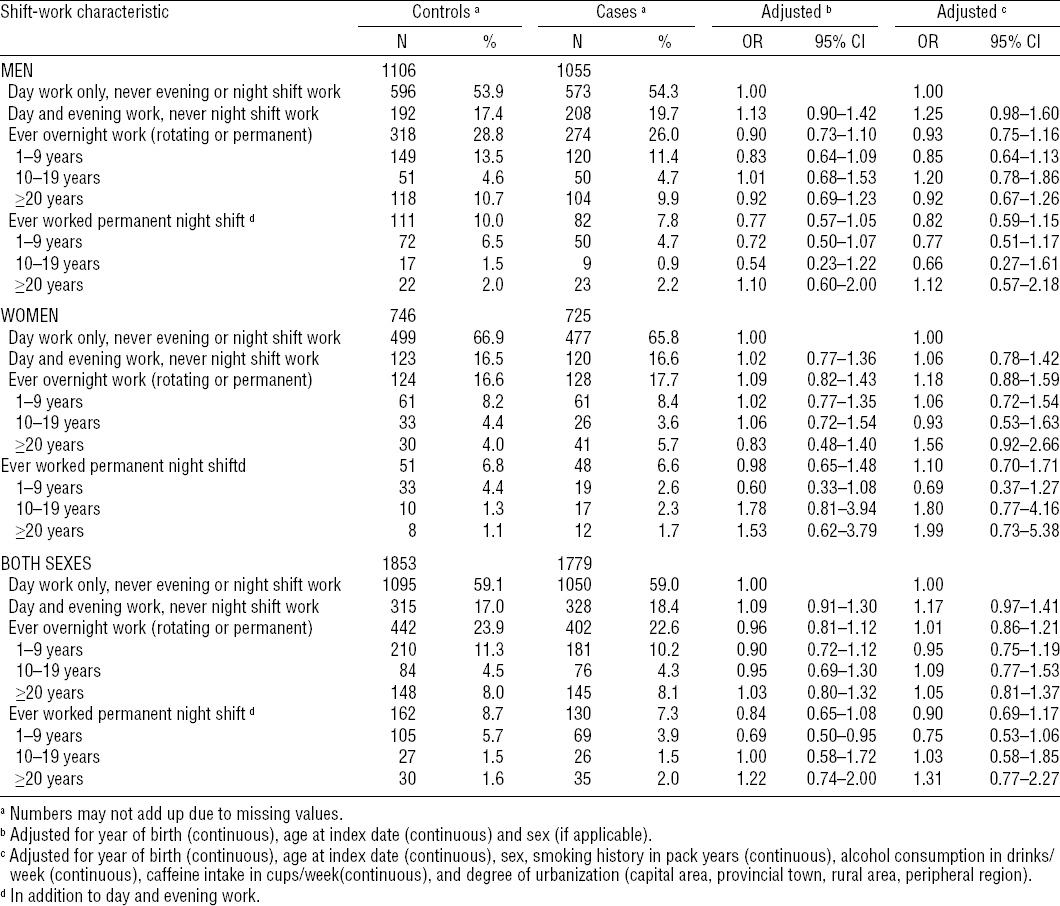
We also examined the associations between duration of shift work and PD risk (table 2). Compared to those who had always worked only during the day, the number of years working overnight shifts (ie, either rotating or permanent night shift work) was not associated with PD risk (eg, ≥20 years of overnight shifts, multivariate OR 1.05, 95% CI 0.81–1.37). Similarly, among those who worked permanent night shifts only, PD risk was not different compared to that of day shift only workers (eg, ≥20 years permanent night shift work, multivariate OR 1.31; 95% CI 0.77–2.27). Though based on small numbers in the longer duration categories, analyses stratified by gender were similar among men and women (table 2).
Lastly, in analyses stratified by smoking status, OR also remained similar; they were 0.93 (95% CI 0.74–1.17) among ever smokers and 1.12 (95% CI 0.84–1.50) among never smokers for any type of overnight work, men and women combined.
Discussion
Overall, we did not see any significant associations between night shift work and PD in our study. Neither rotating nor permanent night work schedules were significantly associated with PD risk if these schedules were adopted for periods < or >20 years.
The reasons for dopaminergic neuron death in PD pathogenesis are largely unknown, but substantial evidence suggests an important role of oxidative stress (7, 8). Melatonin is a potent endogenous antioxidant, which has been hypothesized to be beneficial in the prevention and treatment of PD based on experimental data (11–14). Previous epidemiological studies have suggested that working night shifts may increase risks of different cancers among both women and men (5, 22), possibly in part by suppressing circulating levels of melatonin (15, 16). Working night shifts disrupts the normal physiological circadian rhythm and has a wide range of physiological, psychological, and social impacts on shift workers (4). However, to our knowledge, there has only been one previous epidemiological study that investigated a potential association between working on rotating night shifts and the risk of PD. In this prior prospective cohort study of nurses with 181 PD cases only, working for more years on night shift was associated with a lower risk of PD after adjusting for age and smoking (6). In this study, years of night shift work was positively associated with smoking status; however, the fact that never smoking shift workers also had lower PD risk argued against the possibility of substantial residual confounding by smoking. A possible mechanistic explanation of this inverse association could be that both lower melatonin and shift work have been related to modest increases in plasma concentrations of estradiol and uric acid (23, 24), both of which may be protective against PD (25, 26). Another difference of note is the lower average of duration of sleep among PD cases in our study, compared to the positive association between longer sleep duration and PD risk in the Chen et al study (6).
The current study is only the second epidemiological study to examine a potential association of night shift work with PD risk and must be considered exploratory. In our study, neither rotating nor permanent night work schedules were associated with PD risk, regardless of duration of overnight work.
A major strength of the study is its size and the detailed medical record review, which allowed us to exclude non-idiopathic and erroneously registered PD cases. A potential limitation of note in this retrospective case-control study is recall bias. However, it appears unlikely that patients would have been less likely to report night shift work or the controls more likely to do so. Nevertheless, the study population was elderly, and eliciting information on a lifelong history of employments in this population will likely have been somewhat inaccurate, leading to some degree of non-differential misclassification of exposure. In general, as in the analysis by Chen et al (6), most studies evaluating the association between shift work and adverse health outcomes including ours are hampered by crude exposure measurements. Another limitation of our study is that hospital registers captures only roughly 80% of all eligible PD cases living in Denmark, since a small fraction of PD patients remains treated in private practice only and never visit specialty clinics that would report them to the register. Due to the imposed eligibility criteria of our case selection, cases in our study are likely somewhat younger and less severely ill than all prevalent cases in Denmark combined, thereby possibly limiting the external validity of our data. Nonetheless, given the high fraction of patients eligible for our case control study, we would expect them to be quite representative of all Danish PD patients. Finally, the modest participation rate among cases (81%) and controls (53%) could have biased the results if a history of night shift work was associated with willingness to participate, which appears unlikely given that most participants were no longer in the workforce. Lastly, even though we controlled for known potential confounding factors, there may still be uncontrolled confounding, such as stress or other lifestyle differences. Yet, whether to treat such factors, eg, cortisol levels, as confounding or intermediate factors would need to be considered.
In conclusion, our study did not provide support for an association between night shift work and risk of PD. Larger studies with improved exposure assessments are needed to further delineate the potential effects of shift work on PD risk.