Together, oral cavity cancer (OCC) and pharyngeal cancer (PhC) are the eighth most frequently occurring neoplasms worldwide, giving rise to 482 000 new cases in 2008 (1). The main risk factors for OCC and PhC are (excessive) alcohol consumption and all forms of tobacco use, while fruits and vegetables have been associated with a decreased risk (2). Occupational carcinogens, such as polycyclic aromatic hydrocarbons (3) and solvents (4), may also be involved in the etiology of both cancers, but the strongest suggestion of a possible association focuses on asbestos exposure (3, 5, 6). There are, however, no supportive data from animal studies and a clear exposure–response relation for asbestos exposure is lacking (5). As such, there is still debate whether asbestos exposure increases the risk of developing OCC and PhC, and if so, whether risk increases with increasing level of cumulative exposure. Besides questions on the strength of a possible association, there are additional questions relating to (i) the association with OCC and PhC separately, as they have often been combined in previous research; (ii) possible confounding by other risk factors, such as smoking and alcohol consumption, as most previous studies were performed in an industry-setting and were unable to adjust for these factors; and (iii) the presence of an interaction between exposure to asbestos and smoking in relation to the risk of OCC and PhC, as has been suggested for other asbestos-related cancers (5, 6).
Population-based studies are well-suited to address these questions given their overall wide range in exposure levels, possibility to control for potential confounders, and large size. The prospective Netherlands Cohort Study (NLCS) is a population-based study, which started in 1986 among 120 852 men and women of the general population (7). Within the framework of the current investigation, we had the following objectives: (i) to investigate the association between occupational asbestos exposure and risk of OCC and PhC both separately and combined, paying special attention to the existence of an exposure–response relation and the influence of potential confounding on risk estimates; (ii) to study the presence of an interaction between asbestos and smoking in relation to the risk of OCC and PhC, both separately and combined.
As the proportion of long-term employed women was rather low (resulting in <1% being occupationally exposed to asbestos), this study was conducted only among men.
Methods
Study population and cancer follow-up
The study design and data collection strategies for the NLCS have been described in detail previously (7). In brief, the NLCS started in September 1986 when 58 279 men and 62 573 women aged 55–69 years, originating from 204 municipalities in the Netherlands with computerized population registries, were enrolled in the cohort (response rate 35.5%; 34.5% among men and 36.6% among women). At baseline, participants completed a self-administered questionnaire on dietary habits and lifestyle, occupational history, and other potential risk factors for cancer (7). For reasons of efficiency in questionnaire processing and follow-up, the case-cohort approach was used (8). Incident cases were enumerated from the entire cohort, whereas the accumulated person-years at risk in the entire cohort were estimated from a random subcohort of 5000 subjects (2411 men and 2589 women), selected immediately after baseline.
This subcohort is being followed-up for vital status information while the entire cohort is being monitored for incident cancer by annual record linkage to the Netherlands Cancer Registry and the Dutch Pathology Registry (PALGA) (9, 10). For current analyses, a total of 17.3 years of follow-up (baseline to December 2003) was available. Completeness of incident cancer coverage was estimated to be almost 100% (11) as was the completeness of follow-up of the subcohort. The institutional review boards of the Netherlands Organization for Applied Scientific Research TNO (Zeist) and Maastricht University approved the NLCS.
End points for this study were incident, microscopically confirmed OCC and PhC cases classified by anatomic site or histological type, as defined by the International Classification of Diseases for Oncology, Third Edition. Tumor assignment followed the International Head and Neck Cancer Epidemiology Consortium (INHANCE) collaboration classification (12) and consisted of the following categories: (i) oral cavity (includes lip, tongue, gum, floor of mouth, and hard palate): codes C00.3–C00.9, C02.0–C02.3, C03.0, C03.1, C03.9, C04.0, C04.1, C04.8, C04.9, C05.0, C06.0–C06.2, C06.8, and C06.9; (ii) pharynx, consisting of (a) oropharynx (includes base of tongue, lingual tonsil, soft palate, uvula, tonsil, and oropharynx): codes C01.9, C02.4, C05.1, C05.2, C09.0, C09.1, C09.8, C09.9, C10.0–C10.4, C10.8, and C10.9; and (b) hypopharynx (includes pyriform sinus and hypopharynx): codes C12.9, C13.0–C13.2, C13.8, and C13.9; 3) oral cavity, pharynx unspecified or overlapping: codes C02.8, C02.9, C05.8, C05.9, C14.0, C14.2, and C14.8. As most tumors in our study originated from squamous cell tissue (89.6%), analyses were restricted to cases with squamous cell carcinomas.
Other than skin cancer, all prevalent cases at baseline were excluded, leaving 2336 male subcohort members, 71 OCC and 74 PhC cases (50 oropharynx and 24 hypopharynx). Cases that could not be classified as oropharynx, hypopharynx, or oral cavity (N=2) were included only when analyzing OCC and PhC together (OCPC; N=147). Due to the low number of oro- and hypopharyngeal cancer cases, no subtype analyses were performed. Subjects without any, or only uncodable, information on occupational history or who never worked professionally were omitted from the analyses. As a result, 2101 male subcohort members, 63 OCC, 67 PhC, and 132 OCPC cases were available for analysis after 17.3 years of follow-up.
Occupational exposure assessment
Information on lifetime occupational history until 1986 was obtained from the questionnaire completed on study enrolment. Questions concerned the job title, name and type of the company, products made in the department, and period of employment. For all subjects, the job code was assessed for each of the maximally five occupations subjects could enter between starting work and 1986.
Job-exposure matrix
We applied two job-exposure matrices (JEM), a general population JEM from the Netherlands (DOMJEM) and a Finnish JEM (FINJEM) as described previously (13), in order to provide insight into the methodological uncertainty associated with choice of JEM with respect to asbestos exposure. DOM-JEM and FINJEM showed moderate agreement amongst each other and rather similar agreement with case-by-case expert assessment. Briefly, occupational exposure experts in the Netherlands developed DOMJEM for application in general population studies. It contains a combined measure of the probability×intensity of exposure, which is ordinal (no, low, or high exposure) with a weighting of respectively 0, 1, or 4 (14). FINJEM was constructed for exposure assessment in large register-based studies and is based on both expert assessment and exposure measurements. It contains continuous estimates of the prevalence and intensity of exposure both separately and combined, and contains a time axis (15). Although FINJEM was constructed for Finland, exposure estimates were not adapted to Dutch occupational circumstances before application in the NLCS.
Asbestos exposure variables
Several exposure variables were defined, which we have described in more detail elsewhere: ever versus never occupationally exposed to asbestos (yes/no), cumulative exposure [CE; a combined measure of the probability (P), intensity (I), and duration (years) of exposure, measured in fiber-years/ml (f-y/ ml) (FINJEM) or unit-years (DOMJEM)], ever versus never highly exposed to asbestos (yes/no), and duration of high exposure (years) (16).
Participants were classified into never-exposed subjects and tertiles of those exposed to asbestos based on the distribution among the subcohort for the CE (never exposed=reference group) and for the duration of high exposure (never highly exposed=reference group). Continuous variables were also used; for the CE, an increment of 1 unit-year (DOMJEM) or 1 f-y/ml (FINJEM) was used.
For the percentage of the population for whom some information on occupational history could not be coded, exposure to asbestos was set to zero for the period with unclear exposure.
Statistical analysis
Cox proportional hazards models were used to estimate age-adjusted as well as multivariable-adjusted hazard ratios (HR) and corresponding 95% confidence intervals (95% CI).
The total person-years at risk were estimated from the subcohort (17), and we estimated standard errors using a robust covariance matrix estimator to account for increased variance due to sampling the subcohort from the entire cohort (18).
The covariates included in the multivariable-adjusted models were either a priori-selected risk factors based on the literature or variables that changed the age-adjusted regression coefficients by ≥10% (using a backwards stepwise procedure). Smokeless tobacco has not been included in the multivariable-adjusted model as the total number of cases that used this form of tobacco was too low (N=4) to be of any influence as a confounder in the NLCS. Polycyclic aromatic hydrocarbons and solvents have also not been included in the final model as these exposures hardly changed the age-adjusted regression coefficients. For all endpoints, the full covariate model consisted of smoking status (never/former/current), number of cigarettes smoked per day (centered variable), years of smoking cigarettes (centered variable), socioeconomic status (by level of education; lower vocational, secondary and medium vocational, and higher vocational/university), alcohol consumption, and consumption of vegetables. Information on consumption of vegetables was gathered in the dietary section of the baseline questionnaire. This section consisted of a 150- item semi-quantitative food-frequency questionnaire, which concentrated on habitual consumption during the year preceding the start of the study.
All covariates were entered into the models as continuous variables, except for smoking status and socioeconomic status. To enable comparison, the models adjusted for age and family history of cancer were restricted to subjects included in the multivariable-adjusted analyses (ie, with no missing values on confounding variables), which left 1858 subcohort members, 58 OCC, 53 PhC, and 113 OCPC cases for analyses. For each analysis, scaled Schoenfeld residuals were used to test the proportional hazards assumption (19). Trends for all subjects were evaluated with the Wald test by assigning subjects the median value for each level of the categorical variable among the subcohort members, and this variable was entered as a continuous term in the Cox regression model.
Furthermore, we tested for a possible interaction between occupational exposure to asbestos (yes/no) and smoking status (never/former/current) in relation to OCC, PhC, and OCPC. As testing for departure from additivity was not possible due to low numbers, we only studied statistically significant departure from multiplicativity by including an interaction term in the Cox regression model. All tests (2-tailed) were performed using Stata, version 10 (Stata Corp, College Station, TX, USA), and differences were regarded as statistically significant at P<0.05.
Results
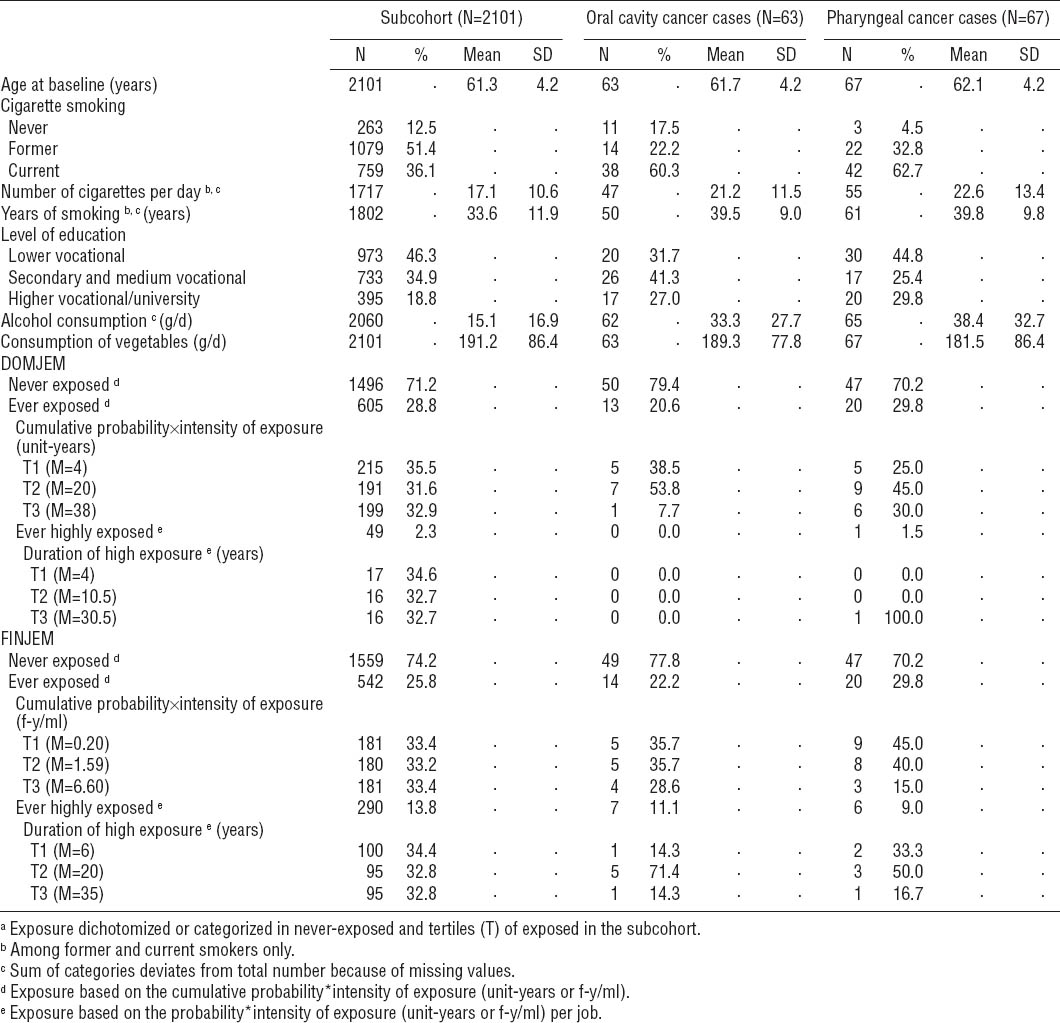
The distribution of occupational exposure to asbestos and potential confounders among male subcohort members and cancer cases in the NLCS is presented in table 1. Overall, OCC and PhC cases more often smoked cigarettes, smoked more cigarettes per day and for a longer period of time, and consumed more alcohol per day than subcohort members. OCC and PhC cases generally had a higher socioeconomic status than subcohort members. According to both JEM, OCC cases were overall less often exposed to asbestos than subcohort members, while PhC cases were more often exposed. As the number of highly exposed subjects was very low, certainly for DOMJEM, no further results will be presented for ever versus never highly exposed and the duration of high exposure.
Table 1
Distribution of potential confounders and asbestos exposure a among male subcohort members and cancer cases in the Netherlands Cohort Study (NLCS) 1986–2003. [SD=standard deviation; DOMJEM=Dutch general population job-exposure matrix; FINJEM=Finnish JEM; M=median]
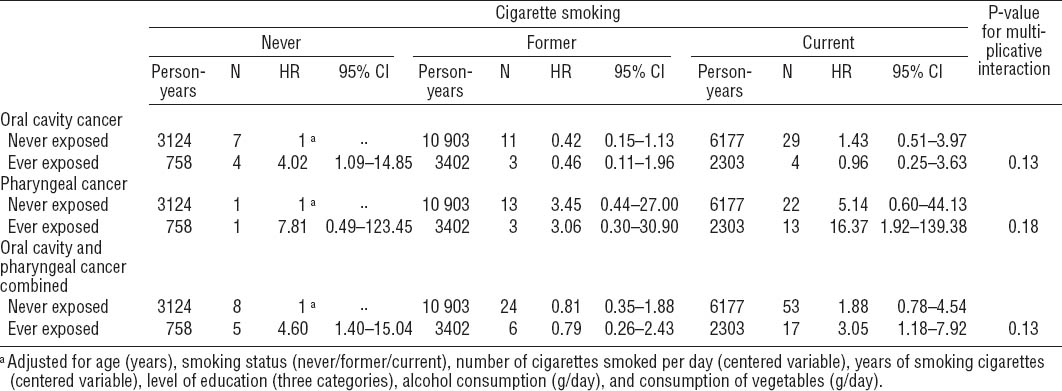
Overall, most asbestos exposure variables showed no association with OCC, PhC, and OCPC (table 2). Adjusting for potential confounders was generally of minor influence, except for alcohol consumption and socioeconomic status, which increased the HR of PhC and OCPC. Therefore, only multivariable-adjusted results are presented below.
Table 2
Hazard ratios (HR) and 95% confidence intervals (95% CI) for oral cavity and pharyngeal cancer both separately and combined, for categories of asbestos exposure a estimated with a general population and Finnish job-exposure matrix (DOMJEM and FINJEM) in the Netherlands Cohort Study (NLCS), 1986–2003. [M=median]
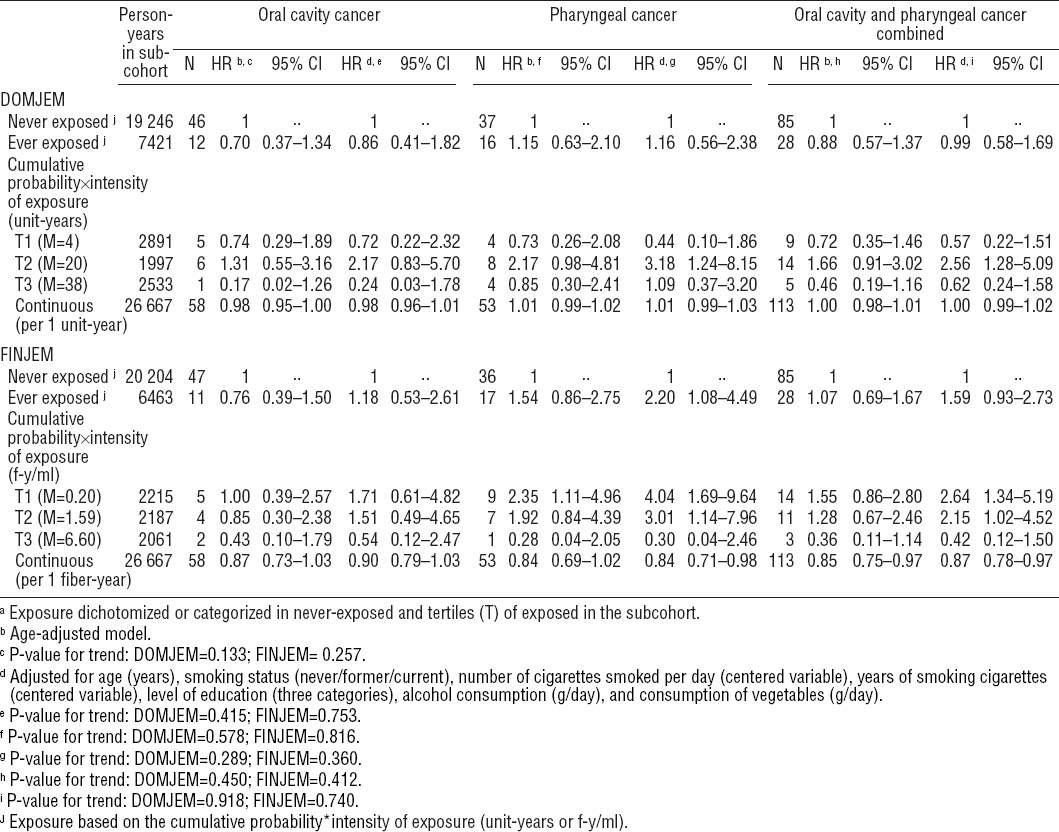
For OCC, no associations were observed when using DOMJEM or FINJEM for ever versus never exposed [HR 0.86 (95% CI 0.41–1.82) and HR 1.18 (95% CI 0.53–2.61), respectively].
For PhC, an elevated risk was observed for ever versus never exposed when using FINJEM [HR 2.20 (95% CI 1.08–4.49)], but not when using DOMJEM [HR 1.16 (95% CI 0.56–2.38)]. No trends of increased risks with higher cumulative exposure could be demonstrated when using DOMJEM or FINJEM. Certainly when using FIN-JEM, risk in tertile 3 of cumulative exposure was lower [HR 0.30 (95% CI 0.04–2.46)] than in tertiles 1 and 2 [HR 4.04 (95% CI 1.69–9.64)] and [HR 3.01 (95% CI 1.14–7.96)].
For OCPC, results showed patterns similar to those observed for PhC, with tertiles 1 and/or 2 of the cumulative exposure showing an association [tertile 2 when using DOMJEM: HR 2.56 (95% CI 1.28–5.09) and tertiles 1 and 2 when using FINJEM: HR 2.64 (95% CI 1.34–5.19) and HR 2.15 (95% CI 1.02–4.52), respectively]. The P for trend was non-significant for any of the cancer endpoints.
For ease of presentation, we will only present interaction results for FINJEM (table 3). Although the stratum-specific HR may be suggestive of a negative interaction between asbestos and smoking for OCC, none of the cancers showed a statistically significant interaction. Analyses with DOMJEM revealed no interactions (data not shown).
Discussion
This study showed no convincing evidence of an association between asbestos and risk of OCC, PhC, and OCPC, as an exposure–response relation was lacking and the ever versus never exposed estimates were inconsistent across both JEM. Nevertheless, some HR of PhC and OCPC were increased. Adjustment for especially alcohol consumption and socioeconomic status further increased HR of PhC and OCPC. Although the strataspecific HR may be suggestive of a negative interaction between asbestos and smoking for OCC, none of the cancers showed a significant interaction.
Oral cavity cancer
There are only few studies that have investigated the asbestos-related risk of OCC. These studies showed non-significantly increased risks (20, 21) as well as decreased risks (22, 23), with a recent meta-analysis revealing relative risk (RR) estimates of HR 1.13 (95% CI 0.81–1.57) and HR 1.15 (95% CI 0.84–1.57) for low and high exposure, respectively, based on five studies (3). Risk estimates in our study are in line with the results of the meta-analysis.
Pharyngeal cancer
For PhC, risk estimates of around one (21, 22) as well as increased risks have been reported after asbestos exposure in both overall PhC (23) and hypoPhC (20, 24). Our study reported multivariable-adjusted increased HR of PhC for ever versus never exposed to asbestos (HR 2.20, 95% CI 1.08–4.49) when using FINJEM, but a trend of increased risks with higher cumulative exposure could not be demonstrated. Furthermore, given that results were not robust against the use of different JEM, one could conclude that our study showed no convincing evidence of an association between asbestos and PhC. However, the number of cases in our study was small. A meta-analysis also showed increased risks of PhC without evidence of an exposure–response relation [relative risk (RR) of 1.26 (95% CI 0.96–1.66) and 1.27 (95% CI 0.98–1.66) for low and high exposure, respectively] (3), whereas a recent large case–control study observed an exposure–response relation (23). Therefore, the rather consistent observation of a possible association between asbestos and PhC could be more than a mere chance finding and warrants further research in studies with a larger number of cases.
Oral cavity and pharyngeal cancer combined
Because other studies mostly combined OCC and PhC, we also studied both cancers together (OCPC), notwithstanding the possibility that the asbestos-related risk may differ for both cancers. We found increased risks for ever versus never exposed and the CE, similar to but less strong than for PhC. Results of previous cohort studies are rather consistent and show modestly increased risks with a meta-RR of 1.44 (95% CI 1.04–2.00), while case–control studies are rather limited in number and show inconsistent results (5). Data on exposure–response patterns from both types of study are limited and tend towards lower risks for the more extreme exposures (5) as was the case in the present study. Another study using FINJEM found no exposure–response association either (4), but a meta-analysis stratifying results on exposure circumstance showed a RR of 1.63 (95% CI 1.27–2.09) for asbestos miners and millers with the highest exposures (3). Due to the small number of cases, we were not able to run the analyses for the (prolonged) highly exposed subjects and test this result. Moreover, exposure levels of the miners and millers were probably much higher than in the NLCS.
Contrary to lung cancer, there is almost no epidemiological or experimental evidence addressing whether interaction may be present between asbestos and tobacco smoking in the development of OCC, PhC, and OCPC (5). Previous studies found no significant interaction for overall PhC (23) and hypoPhC (24). Our study also found no significant interaction for any of the cancers. The number of cases was small, however, and non-differential asbestos exposure misclassification, due to using JEM, may have hampered finding significant results.
The present study suffers from some of the same limitations as previous studies, ie, a small number of cases and suboptimal characterization of asbestos exposure due to using JEM and the fact that information on occupational history was gathered at baseline in 1986 while study subjects were followed-up to December 2003. Nevertheless, our results are of importance for future meta-analyses given the limited number of studies on this subject. Moreover, the strengths of our study include the prospective design, the long, nearly complete follow-up, and the possibility to adjust for several lifestyle confounders. Evidence comes mainly from occupational cohorts that do often not allow for adjustment for potential confounders. Since HR of PhC and OCPC increased after adjustment for especially alcohol consumption and socioeconomic status, taking lifestyle factors into account may be important when studying these cancers. Furthermore, while our JEM-based exposure assessment possibly entailed non-differential exposure misclassification resulting most likely in bias towards the null value, a previous study in the NLCS using both DOMJEM and FINJEM was able to demonstrate the well-known associations between asbestos and cancers of the pleura and lungs (16). In addition, as study subjects were between 55–69 years of age at the start of the study in 1986, the amount of exposure misclassification resulting from the fact that we had no information on occupational history from 1986–2003 will probably be limited.
In conclusion, this study showed no convincing evidence of an association between asbestos and OCC, PhC, and OCPC risk as an exposure–response relation was lacking and results were not robust against the use of different JEM. However, increased HR of PhC and OCPC were observed in this study as well as in previous studies and warrant further research.