Musculoskeletal disorders (MSD) are common causes of disability retirement from work (1, 2). Nearly a third of disability retirement in the Nordic counties are due to MSD, highlighting their public health and societal significance (1, 2). Previous prospective cohort studies found that physical workload (3, 4), occupational class (5), chronic pain (6), psychosocial factors (7), mental stress (4), self-perceived physical health (8), and leisure-time physical activity (1) predict disability retirement due to MSD.
It is important to identify individuals at increased risk of work disability to be able to target healthcare interventions. A risk screening tool uses a number of worker’s characteristics and risk factor profiles to predict the risk of future work disability. Some risk factors of disability are challenging and costly to measure accurately such as lumbar disc disorders or carpal tunnel syndrome. For a risk screening tool to be generally applicable, it should be limited to readily available or assessable information such as sociodemographic factors, working conditions, and pain symptoms. To the best of our knowledge, no screening tool has been developed to predict the risk of disability retirement due to MSD among the general working population.
Three important aspects of a risk screening tool are its discriminatory performance, calibration, and validation (9). Discrimination refers to the ability of a tool to accurately classify individuals with and without the endpoint of interest (eg, disability retirement) (9). Calibration refers to the level of agreement between observed and predicted risk. If the tool under- or over-estimates the risk, calibration is poor. Furthermore, over-fitting or over-adjustment is a common problem where the construction of a prediction model is based on a small sample, few endpoints, or inclusion of too many predictors. An overfitted screening tool is likely to underestimate the risk among low-risk individuals and to overestimate the risk among high-risk individuals. Poor discrimination is considered a more serious failing than poor calibration (10). Evaluation of the discriminatory performance of a risk screening tool is the most important consideration in its development. Moreover, a risk screening tool should be internally and externally valid. Internal validity refers to precision or reproducibility of the risk and external validity helps determine generalizability of a screening tool to other populations.
The objective of this study was to develop and validate a risk screening tool using a points system to predict the risk of future disability retirement due to MSD.
Materials
Development population
The development sample consisted of the population of the Health 2000 Survey (2, 11). In this survey, a representative sample of individuals aged ≥30 years living in Finland in 2000–2001 was recruited using a two-stage cluster sampling design. The sample was stratified according to five university hospital regions. From each region, 16 healthcare districts were sampled as clusters.
The sample included 7977 individuals aged ≥30 years. Of these, 6986 (87.6%) were interviewed and 6354 (79.7%) participated in the health examination (11). For the purposes of this study, we included participants who held a full- or part-time job during the preceding 12 months at baseline in 2000–2001 (N=4201). Participants aged ≥60 (N=97) were excluded from the analysis. Participants with missing information on predictors (N=428) were excluded, leaving 3676 subjects eligible for the analyses.
All participants signed a written informed consent, and the Ethics Committee for Epidemiology and Public Health of the Hospital District of Helsinki and Uusimaa, Finland, approved the study. The data on deaths during the follow-up period were obtained from Statistics Finland and data on disability pensions granted between 2001 and December 2011 were obtained from the national registers of the Finnish Centre for Pensions. The Health 2000 Survey data were linked to data on deaths and disability pensions using the unique identification numbers assigned to each citizen in Finland.
Outcome
In Finland, work ability has to be reduced by at least 60% during one year before assessment of the eligibility for disability retirement (12). Having a diagnosed MSD is a necessary but not a sufficient condition for disability pension because other factors such as age, education, and working conditions are considered when granting a disability pension (12). The national pension register classifies the main diagnoses of chronic illnesses according to ICD-10 codes. The register includes the primary and secondary causes of work disability. The outcome of this study was permanent or temporary disability retirement due to MSD as a primary cause of work disability.
Predictors
Sociodemographic and lifestyle factors
The home interview elicited information on age, sex, marital status, education, and smoking. Level of education was defined as low (basic comprehensive school certificate), medium (upper secondary or vocational school diploma), and high (college or university degree). Information on the nature, frequency and duration of participation in leisure-time physical activity, number of hours of sitting during weekdays and weekends, and walking or cycling to and from work was collected. Sedentary lifestyle was defined as participation in leisure-time physical activity for <2–3 times per month, and reading, watching TV or doing other activities that do not demand moving or straining physically during leisure time. As part of the health examination, body weight and height, and waist and hip circumferences were measured.
Occupational factors
The presence of the following physical exposures in the current job was assessed by the home interview (13, 14): (i) a heavy physical work; (ii) work demanding the hands above the shoulder girdle; (iii) manual handling of loads >5 kg; (iv) manual handling of loads >20 kg; (v) work demanding high handgrip force; (vi) repetitive movements of the hands or wrists; (vii) work with a vibrating tool; (viii) using keyboard; (ix) work demanding kneeling or squatting; (x) work requiring driving a car, tractor or other work machine; (xi) work demanding standing or leaning forward without support; (xii) work requiring sitting ≥5 hours/day; and (xiii) work demanding standing or walking ≥5 hours/day.
The following work-related psychosocial factors were assessed by the home interview or a questionnaire: (i) social support, job demands and control (15); (ii) regular night job, 2- and 3-shift employment, and number of working hours per week; (iii) conditions and atmosphere of workplace; (iv) threats involved in the work such as mental violence, bullying, becoming unemployed, or being laid off; and (v) burnout (16).
Perceived current physical and mental work ability were also assessed by two questions (17).
Musculoskeletal pain and disorders
The home interview included questions whether the individual had suffered, according to a physician’s diagnosis, neck or back disorders, rheumatoid arthritis or other inflammatory joint diseases, osteoarthritis, osteoporosis, fractures, pain in the neck, shoulder, or the back limiting daily activities in the past five years, a history of carpal tunnel release, surgery for neck or back disease, and a history of neck or back disease treated by a physician. Information on the location of osteoarthritis and fractures was also collected. As a part of the health examination, a specially trained nurse carried out a symptom interview. The participants were asked whether they had experienced pain, ache or motion-related soreness during the last 30 days or during the last 7 days in the following body areas: the neck, shoulder, upper arm, elbow, wrist, fingers, back, hip, knee, ankle or foot. We defined multisite musculoskeletal pain as having pain in at least two of four locations (neck, back, upper extremity, and lower extremity) during the preceding month or during the preceding seven days (18). Moreover, at the health examination, a physician carried out a standardized clinical examination and, according to pre-set criteria, diagnosed the following MSD: rotator cuff tendinitis, bicipital tendinitis, lateral and medial epicondylitis, carpal tunnel syndrome, chronic shoulder syndrome, chronic neck syndrome, chronic back syndrome, sciatica, rheumatoid arthritis, and knee and hip osteoarthritis.
Other predictors
The home interview included questions on depression, anxiety, psychosis, and substance use. Mental health was also assessed at the health examination using the computerized version of the Composite International Diagnostic Interview (CIDI) (19). The presence of depressive symptoms was also assessed by the Beck Depression Inventory (20). Self-rated health was inquired by a single question with five response alternatives: excellent, very good, good, fair, or poor. Fasting serum samples were drawn for the assessment of triglyceride, total cholesterol, low and high density lipoprotein cholesterol, and sensitized C-reactive protein.
Validation population
The Helsinki Health Study was conducted among employees of the City of Helsinki aged 40–60 years old in 2000–2002 (N=8960, response rate 67%, 80% women) (21). The data were linked to disability pension and mortality data for 11 years follow-up. The register linkages were made for those with a written consent for combining their survey responses with these register data (74%). The final population comprised 6391 participants with data on all variables of interest. Analyses of non-response and consent-giving suggest that the data broadly represent the target population, although men, people with long sickness absence, and those with a lower socioeconomic position were slightly overrepresented among non-responders and those not providing (or slightly less likely to provide) consent for register linkage (21, 22). The ethics committees of the health authorities of the City of Helsinki and the Department of Public Health, University of Helsinki, approved the study.
Outcome
The outcome in the validation analyses was temporary or permanent disability retirement due to MSD as a primary cause of work disability, similar to the development population.
Predictors
Level of education was reported in five categories from basic education to university/college degree. Higher level of education was defined as having a college or university degree. The experience of pain was assessed using a single item asking whether the participant currently had any pain or aches (“yes”/”no”). Participants with a positive response also responded to the question about the location of their pain. Four locations were used: the neck or shoulders, low back, arms, and legs. Multisite pain was defined as having pain in at least two out of the four locations. Participants with current pain were asked to rate the severity of their pain limiting daily activities. We dichotomized the responses into no and yes. Finally, the participants were enquired whether a physician had ever told them that they have rheumatoid arthritis or osteoarthritis.
Statistical analysis
In the development population, nearly 10.4% of the participants had missing data on one or more of the predictors, particularly on multisite pain and pain limiting daily activities. The missing data on the predictors were imputed using the method of multiple imputation by chained equations described by van Buuren et al (23) and implemented in the Stata software by Royston (24). For this, we included also auxiliary and outcome variables and created 20 datasets using the Stata ice command (Stata Corp, College Station, TX, USA). The estimates produced by multiple imputation-based analyses were differed only slightly from those produced by complete-case analyses. We therefore constructed a screening tool based on complete-case analyses.
A survey data analysis was conducted by using Stata’s svy prefix command (25). Participants who died during follow-up and those who were no longer at risk of disability retirement due to old age pension were censored. Multiplicative interactions were tested by including product terms in the multivariable models between age and sex, between age and pain limiting daily activity, between multisite pain and depression, between smoking and abdominal obesity, between physical inactivity and obesity, and between forward bending of the low back and abdominal obesity.
We tested for violation of proportional hazards assumption, and the index was developed in the absence of violation. After Cox regression model we calculated Gönen & Heller’s K concordance statistic (26). For Cox regression, Harrell’s C is an equivalent to the receiver operating characteristic (ROC) curve in logistic regression and is based on the proportion of all usable subject pairs in which the predictions and outcomes are concordant (27). Because censoring was present in the current study, we therefore preferred Gönen & Heller’s K concordance statistic to Harrell’s C (26). The area under the curve (AUC) and Gönen & Heller’s K concordance statistic reflect the ability of the score to discriminate between individuals with and without disability retirement. An AUC of 0.50 indicates no discriminatory ability and 1.0 indicates perfect discriminatory ability. An AUC of 0.6–0.7 indicates poor discrimination, 0.7–0.8 fair, 0.8–0.9 good, and 0.9–1.0 excellent discrimination (28).
To construct a prediction index, for each predictor we first performed Cox proportional hazards regression model adjusted for age, sex, and education (29). We then conducted six full models for the predictors that remained statistically significant in the first step: (i) sociodemographic and lifestyle factors, (ii) musculoskeletal pain or disorders, and fracture, (iii) occupational physical factors, (iv) psychosocial and psychological factors, (v) lipids, and (vi) other predictors including self-rated health, diabetes, physical and mental work ability, and C-reactive protein. Highly correlated variables were not included in the same model. Finally, we performed a full model including all variables that remained statistically significant in the second step. Non-significant predictors were then removed from the full model one at the time until all predictors remained statistically significant. We used the most parsimonious rule to include the predictors in the prediction index to derive an easy-to-use clinical screening tool. The predictors that only slightly improved discriminatory ability of the model were removed one at the time. We used also Akaike information criterion (AIC) and Bayesian information criterion (BIC) of Cox model to examine whether the inclusion or exclusion of an additional predictor improved the prediction model. Smaller AIC and BIC values indicated better models.
We constructed a prediction index using a weighted sum of the predictors in the final parsimonious Cox model where the weights are the regression coefficients. Finally, we smoothed the estimates for age in the final Cox model by using cubic splines (30). The results of cross-validation were also used to give the best point to each worker’s characteristic. We examined the internal validity of the prediction model by using k-fold cross-validation. Cross-validation assesses whether the results of a statistical model is generalizable to other data. It assesses the possibility of overfitting and adjusts for optimism/overfitting in predictive ability of the model (10). We randomly partitioned the dataset into five subsets of similar size. We used one subset as the test set (validation) and the remaining four subsets as training set. The process was repeated five times and each of the five subsamples used once as the validation data. We estimated an average 11-year observed and predicted risks for the different levels of the prediction index. We applied easy-to-use points and calculated sensitivity, specificity, and positive and negative predictive values of different disability risk screening tool cut-off points. We applied four cut-points based on the distribution of the prediction index: the top 20%, 25%, 30% and 60% of risk score. Lastly, we examined the external validity of the screening tool. We used Stata, version 13 for the analyses.
Results
Participants
The characteristics of the development population are reported in supplementary table S1 (web appendix: www.sjweh.fi/show_abstract.php?abstract_id=3684).
Thirty-six percent reported a history of pain limiting daily activities in the past five years, 4% reported knee osteoarthritis, and 1% rheumatoid arthritis. Furthermore, 3% had been operated for a spinal disorder or carpal tunnel syndrome. Thirty percent of disability retirement were granted within 3.7 years of the follow-up period, 50% within 5.9 years and 75% within 8.7 years.
Models adjusted for age, sex, and education
Age, sex, and education independently predicted disability retirement due to MSD. Disability was more common among women, older workers, and less-educated people. After adjustment for age, sex, and education, 39 variables predicted disability retirement due to MSD, whereas 21 factors were not related to subsequent disability retirement (see web appendix for details).
Full models for six groups of risk factors
The following factors statistically significantly predicted disability retirement due to MSD after further adjustment for other covariates: (i) sociodemographic and lifestyle factors: age, sex, education and sedentary behavior; (ii) musculoskeletal disorders: fracture, musculoskeletal pain limiting daily activities, physician-diagnosed chronic spinal disorder, multisite pain, a treated spinal disorder, surgery for a spinal disorder or carpal tunnel syndrome, and arthritis; (iii) occupational physical factors: heavy work, work demanding repetitive movements of the hands, and work requiring hand above the shoulder girdle; (iv) psychosocial and psychological factors: high job demands; (v) lipids: total cholesterol to HDL ratio; and (vi) other predictors: physical work ability.
Final full model
In the final full model, 12 predictors remained statistically significant (table 1). Medium level of education and back osteoarthritis did not predict disability retirement due to MSD. Multisite pain in the past 7 days was a weaker predictor [hazard ratio (HR) 1.52, 95% confidence interval (CI) 1.10–2.09] than multisite pain in the past 30 days (HR 3.69, CI 2.15–6.34).
Table 1
Full and the most parsimonious models. [DR=disability retirement; HR=hazard ratio; CI=confidence interval; K= Gönen & Heller’s K concordance coefficient.]
Excluding sedentary lifestyle, work demanding hands above shoulder girdle, and physical work ability did not change the discriminatory ability of the model. A physician-diagnosed chronic spinal disease was also a weak predictor of disability retirement due to MSD. Moreover, Spearman’s rank correlation coefficients ranged between 0.10–0.19 for a treated spinal disorder, pain limiting daily activities, and multisite pain. However, of those who had reported a spinal disorder treated by a doctor, 98.6% reported also pain limiting daily activities or multisite pain. Lastly, we chose the most parsimonious model of seven predictors to construct the screening tool (table 1). The discriminative ability of the model was 0.821 (0.803 in men and 0.831 in women). The test of proportional-hazards assumption was non-significant (P=0.93), indicating absence of evidence to contradict the proportionality assumption.
Construction of prediction index
Finally, we constructed the screening tool using the model with seven predictors. Sex did not predict disability retirement due to MSD among the participants aged 30–45 years (adjusted HR 1.11, CI 0.59–2.09), while it did so among those aged 46–60 years (adjusted HR 1.89, CI 1.30–2.72). Thus, we gave points to the worker’s age depending on sex and to a high level of education, multisite pain in the preceding 30 days, pain limiting daily activities, arthritis, and surgery for a spinal disorder or carpal tunnel syndrome (table 2). We smoothed the estimates for age by using cubic splines.
A total score was computed by summing all the points for the risk profile. The prediction index ranged between -1–7 and its mean was 1.9±1.5 (table 3). Its cut-off-point was >2 points for top 60% and >3 points for top 30%. Overall, a score of ≥3 (top 30%) had good sensitivity and specificity (table 4, supplementary figures S1–S4, web appendix). Individuals in the top 30% of the risk index were at 29 (CI 15–55) times higher risk of disability retirement due to MSD than those in the bottom 40% (table 3, figure 1).
Table 2
Weights given to predictors included in the prediction index. [HR=hazard ratio; CI=confidence interval; K= Gönen and Heller’s K concordance coefficient.]
Table 3
Hazard ratios (HR) for different cut-off points of disability risk prediction index. [DR=disability retirement; CI=confidence interval; SD=standard deviation.]
Table 4
Sensitivity, specificity and the positive and negative predictive values for the risk predictive tool for cut-off of top 30% of index (score >3) in the development and validation populations.
Figures 1a and b
Cumulative hazard of work disability retirement due to musculoskeletal disorders by three groups of prediction index: (A) development population and (B) validation population.
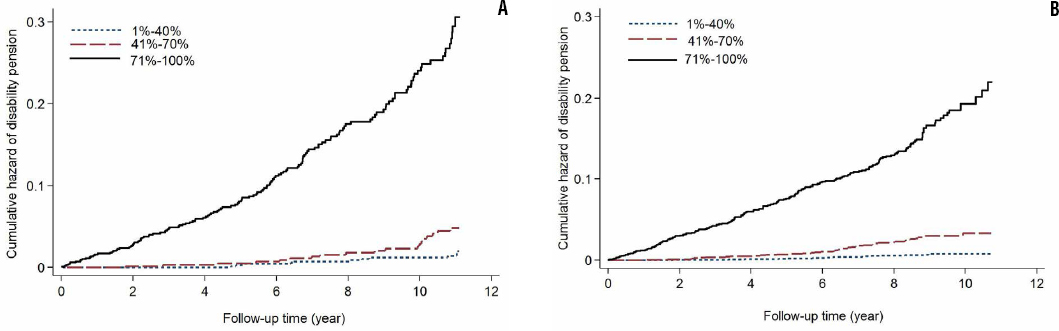
The distribution did not allow using sex-specific top 30% of the risk score. For top 25%, the score was >3 points for men and >3 points for women. The top 25% had good sensitivity and specificity in both men and women (supplementary table S2, web appendix).
The mean score was 0.6±.0 among participants aged 30–40 years, 2.2±1.1 among the 41–50-year-olds and 3.3±1.1 among the 51–60 year-olds. Of the participants, 2.7% of 30–40 year-olds, 38.5% of 41–50-year-olds, and 68.2% of 51–60-year-olds had score ≥3. The corresponding proportions were 0.2%, 12.2% and 46.8% for scores of ≥3.5. The index had high sensitivity and acceptable specificity in all three age groups (supplementary table S3, web appendix). However, false positive cases were highest in individuals aged 30–40 years and lowest in those aged 51–60 years.
Sensitivity analyses
We also constructed the prediction index using age as a continuous variable (a 5-year increase in age). The discriminatory and predictive abilities of the index did not differ from those of the current index using age as a categorical variable. Moreover, adding osteoarthritis of the hand did not improve the discriminatory ability of the index. Physician-diagnosed arthritis (rheumatoid arthritis, knee, or hip osteoarthritis) was a better predictor than self-reported arthritis (adjusted HR 3.24, CI 2.11–5.00 versus 2.55, 95% CI 1.79–3.62). There were 226 self-reported cases but only 125 physician-diagnosed cases. Of the latter, 70.4% also self-reported their arthritis. As our aim was to develop a generally applicable screening tool, we included self-reported arthritis in the prediction index. However, the discriminatory and predictive abilities of the index did not differ after replacing self-reported with physician-diagnosed arthritis.
Internal and external validity
Musculoskeletal pain limiting daily activities predicted disability retirement due to MSD better among 46–60-year-olds (adjusted HR 2.92, 95% CI 2.12–4.02) than 30–45-year olds (adjusted HR 2.00, 95% CI 1.04–3.84). However, the cross validation results and external validation data did not support giving different weight to pain limiting daily activities for the two age groups. The discriminative ability ranged between 0.818–0.827 in five cross validation data. Using age as a continuous predictor, the results of cross-validation did not suggest a need for developing a shrinkage factor to shrink all coefficients with the same factor.
Consistent with the development population, the effect of age was also stronger among women than men in the validation population. Women aged ≥50 but not 40–45 years had higher risk of disability retirement than men. Moreover, current multisite pain had a weaker effect on disability retirement (supplementary table S4, web appendix). The discriminative ability of the model including age, sex, education, multisite pain, arthritis, and musculoskeletal pain limiting daily activities was 0.809 in the validation population. In both development and validation populations, the observed risk matched the predicted risk (supplementary table S5, web appendix). Moreover, the screening tool had a good predictive ability in both populations (table 3, figure 1, and supplementary table S5).
Discussion
In the current study, we developed and tested a novel and easy-to-use risk screening tool for work disability due to MSD. From the factors that predicted disability retirement due to MSDs in a population-based study, we constructed the tool using seven predictors. A score of ≥3 out of 7 had good sensitivity (83%) and specificity (70%). Individuals with a score of ≥3 had a 29-fold (95% CI 15–55) higher risk of disability retirement due to MSD than those with a score of <2.
The risk of disability retirement due to MSD differs by age. It is very low among individuals <41 years, and thus screening for disability due to MSD in this age group does not avail. However, among employees aged 41–50 years, a risk score of ≥3 has high sensitivity and acceptable specificity, likewise for those aged 51–60 years with a score of ≥4. Among employees >50 years, the positive predictive value of the tool increases to 18.5% for top 40% of the index and to 29% for top 20% of the index (supplementary table S3). Moreover, the findings suggest that the screening interval should decrease with increasing age.
The current screening tool was relatively specific to subsequent disability retirement due to MSD. The discriminatory ability of the index was 0.66 for disability retirement due to mental disorders and 0.72 for disability retirement due to somatic disorders, other than mental and MSD. The score of ≥3 had sensitivity of 46%, specificity of 67% and positive predictive value of only 3.4% for disability retirement due to mental disorders, and sensitivity of 59%, specificity of 68% and positive predictive value of 8.3% for disability retirement due to somatic disorders other than mental and MSD. Age, sex and education are common predictors of disability retirement due to these medical conditions (31). It is not therefore unexpected that the tool also has the ability to predict modestly disability retirement due to somatic disorders other than mental or MSD. Moreover, MSD could be a secondary cause of disability retirement among individuals who had been granted a disability retirement due to disorders other than MSD.
Components of the prediction checklist
Obesity, smoking, and several occupational physical and psychosocial factors did not predict disability retirement due to MSD after controlling for musculoskeletal pain or disorders or associated disability. Workload factors predicted disability retirement in the absence of MSD in the model. This indicates that exposure to occupational factors increases the risk of disability retirement by causing MSD. Multisite musculoskeletal pain in the past month was a better predictor than current multisite pain or multisite pain in the past seven days. Musculoskeletal pain limiting daily activities in the past month can also be a better predictor than current disabling pain or disabling pain in the past five years. For screening, we thus suggest asking these pain questions during the preceding 30 days (supplementary table S6, web appendix). We found that a surgery for an MSD is uncommon in the general population. Therefore, the screening tool with only six components can have a predictive ability as good as the tool with seven components.
Strengths and limitations
A strength of our study is that the study samples represent the Finnish working population well. The participation rate of the development population was 85% of the working-aged subjects, which can be considered high. The study population is also well-characterized. The questionnaires, face-to-face interviews, and health examination protocols were mostly selected on the basis of standardized, generally accepted recommendations or nationally established practice (11). In both development and validation populations, we used objective register based data on disability retirement due to MSD. These ICD-10-based data are complete with no losses to follow-up.
However, both studies had some limitations. The development sample was small and 10% had missing information on one or more of the predictors. The response rate for the validation study was 67%, however, the data have been shown to represent the target population (21). The screening tool had good predictive ability in both the development and the validation populations. Limiting the development population to the participants aged ≥40 years (to match with the validation population), 25.4% of 2414 participants had the prediction index >3. The top 25% had a sensitivity of 65.9% and specificity of 77.5%. Its positive predictive value was even higher than that of top 25% in the total sample (17.6%, CI 14.7–20.8% compared with 13.6%, CI 11.4–15.9%).
There are at least two main reasons for better predictive ability of the tool in the development population. First, we had no information on surgery for the disorders of the spine or carpal tunnel release in the validation sample. We tested the external validity of the tool based on six but not seven predictors. Second, multisite pain in the preceding 30 days is more prevalent than current multisite pain or multisite pain in the preceding 7 days. In the validation sample, multisite pain was current musculoskeletal pain rather than pain in the past 30 days. In the development population, we found that multisite pain in the preceding seven days is a weaker predictor of disability retirement due to MSD than multisite pain in the preceding 30 days. Moreover, multisite pain in the preceding 30 days was a stronger predictor among the participants aged ≥40 (HR 3.77, CI 2.07–6.87) than in the total sample.
We included only self-reported items in the tool, making it easy to apply with very low cost. Although validity of self-reported measures can be criticized or questioned, even diagnosis of some conditions such as pain nonetheless need to rely on self-reported symptoms (32). Using clinically verified conditions and objectively measured predictors to comprise the tool may lead to more reliable screening tool but the caveat would be that the tool would not be as easy to use, costs would notably arise and its use would remain limited in clinical practice. Furthermore, most of the clinically verified MSD and objectively measured variables did not predict disability retirement due to MSD after controlling for self-reported symptoms and disorders.
Clinical implications
The risk screening tool is quick, easy, and inexpensive. Furthermore, it is applicable to use by physicians, nurses, employers as well as by employees themselves. Only a simple image of a human figure is necessary to identify body regions for the symptom interview; no other costly equipment is needed. Since the topics are not sensitive, the tool is probably well acceptable by those interviewed. However, its use can only be reasonable for working people whose risk of work disability due to MSD is substantial.
Nevertheless, facilities and a policy on following-up people who screen positively to maintain or improve their working ability must be ensured before any screening action can be started. Thus, the availability of occupational health services is an essential prerequisite. Even then, the screening should be initiated as an experiment with a comprehensive follow-up of experiences. Finally, whether the cost-effectiveness proves sufficient can hardly be evaluated without randomized controlled trials.
Concluding remarks
This easy-to-use screening tool based on self-reported risk factor profiles can help identify individuals at a high risk for disability retirement due to MSD.



