The prevalence of postpartum depression (PPD) is estimated to be 10–15%, but considerably higher rates have been reported across countries (1, 2). PPD imposes potential severe consequences for the mother, child, family and society and is one of the primary causes of maternal mortality in many countries (3, 4). It is therefore considered a global public health problem (5).
The pathogenesis of PPD is complex and multifactorial and involves endocrine, genetic and environmental factors (1, 6). The strongest known risk factors for PPD are prior own or family history of major depressive disorder (1, 6, 7). Other factors shown to be related to the development of PPD include adverse life events, inadequate social support, and low socioeconomic status (SES) (8–10). Identification of potentially modifiable risk factors for PPD is crucial for prevention.
A recent systematic review of 11 longitudinal studies on night work and the risk of depression performed a meta-analysis of the 5 studies with observation periods of ≥2 years revealing some indication of an increased risk of depression among night workers [risk estimate 1.42, 95% confidence interval (CI) 0.92–2.19] (11). Another similar systematic review also including 11 studies used different inclusion criteria. Therefore, only 3 studies contributed to both reviews, and the 8 remaining studies were of cross-sectional design. This review found that night work was associated with an increased risk of depression (relative risk 1.43, 95% CI 1.24–1.64) across sex, night work duration, type of occupation, continent, and type of publication (12). These reviews did not inform whether PPD was included in any of the studies. As pointed out by Angerer and colleagues (11), prior studies on depression in relation to night work are challenged by cross-sectional design, self-reported exposure and outcome, and the possibility of the healthy worker survivor effect, ie, the selection of individuals with poorer health status out of night work (13, 14).
Night work is common among women in the reproductive age. In 2016, around 14% of the female European workers aged <50 years engaged in night work (15). The mechanisms linking night work to the risk of PPD are suggested to involve hormonal dysfunction, inflammation, and sleep disorders (16–25). Sleep disorders are particularly important because they add to the physiological sleep disturbances imposed by pregnancy (25).
We aimed to investigate the association of different dimensions of night work, expressed by frequency and duration of night shifts throughout pregnancy, with the risk of severe PPD. The novel aspect of our study was the use of clinically diagnosed outcome and objective information on pregnancy period-specific exposure.
Methods
Design
Similar to our previously published study (26), we conducted a register-based cohort study with information from the following Danish national registries linked on individual level through the civil registration number: (i) the Danish Working Hour Database (DWHD), a national payroll database covering >250 000 employees in the Danish administrative regions including all public hospital employees from January 2007 to December 2015 (27); (ii) the Danish Medical Birth Registry, which contains information from all births in Denmark since 1973 (28); (iii) the Danish National Patient Registry, which contains data on hospital admissions since 1977 and on outpatients since 1994 (29); and (iv) the Danish Psychiatric Central Research Registry, providing information on psychiatric inpatient treatment since 1969 and on all outpatient treatment since 1995 (30).
Cohort
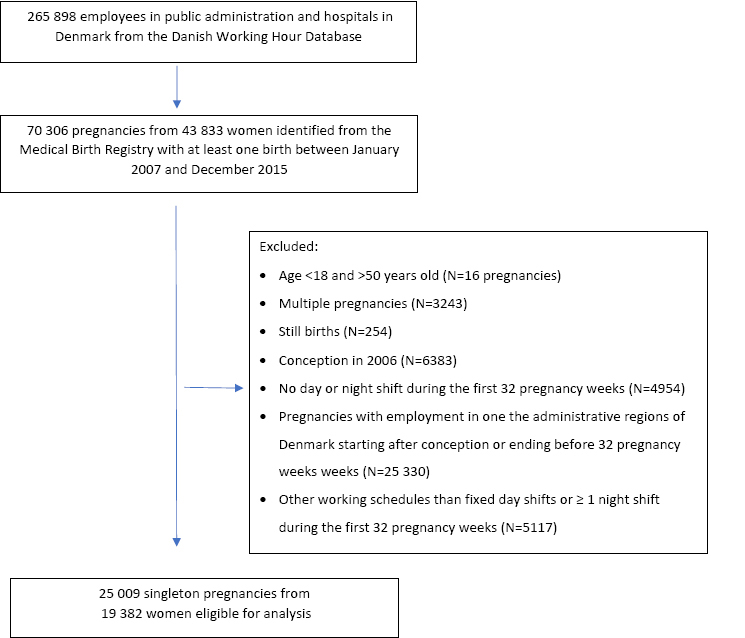
The source population were women from the DWHD who gave birth ≥1 between 2007 and 2015 (N=43 833 women with 70 306 births). We excluded women ≤18 and ≥50 years (N=16 pregnancies); multiple pregnancies (N=3243); pregnancies resulting in still births (N=254); pregnancies conceived in 2006 (N=6383) because they lacked payroll data from conception to January 2007; and pregnancies without ≥1 day or night shift during the first 32 pregnancy weeks (N=4954). To ensure that payroll data was available throughout the first 32 pregnancy weeks, we excluded pregnancies with employment in one the administrative regions of Denmark, ie, registration in the DWHD, starting after conception or ending before 32 pregnancy weeks (N=25 330). Finally, we excluded pregnancies with other working schedules than fixed day shifts or ≥1 night shift during the first 32 pregnancy weeks (N=5117), for example fixed evening shifts, leaving 25 009 singleton pregnancies from 19 382 women eligible for analysis (figure 1).
Exposure
The DWHD provided daily information on the exact time of start and end of all workdays and all types of paid and unpaid leave, job title and place of employment. Shifts during the first 32 pregnancy weeks, including on-call shifts, lasting ≥3 hours were defined as day (start time after 06:00 and end time before 21:00 hours) or night shifts (any start and end time including any duration of working hours 23:00−06:00 hours). They corresponded to 84% of all registered shifts, the rest being early morning or evening shifts. We applied the cut-off of 32 pregnancy weeks because workers in the Danish administrative regions are entitled to pregnancy leave eight weeks prior to their due date. The different dimensions of night work were expressed by the number and duration of night shifts, consecutive night shifts and quick returns as defined below.
Number of night shifts. The cumulated number of night shifts during the first 32 pregnancy weeks was categorized as 0 (fixed day work), 1–8, or ≥9 night shifts (roughly corresponding to ≥1 night shift per pregnancy month on average).
Duration of night shifts. The duration of night shifts was defined as ≤8 hours, 9–12 hours or >12 hours (long night shifts). The categories used for analysis were either working only night shifts ≤8 hours or ≥1 night shift of >12 hours during the first 32 pregnancy weeks.
Consecutive night shifts. Categories of consecutive night shifts were 1 (only single night shifts), 2–3 (≥1 spell of 2–3 consecutive night shifts and no spells of ≥4 consecutive night shifts), or ≥4 (≥1 spell of ≥4 consecutive night shifts) during the first 32 pregnancy weeks.
Quick returns. We defined quick returns as intervals of <11 hours between any type of shifts according to the European Union’s Working Time Directive (31) or of <28 hours after a night shift, as suggested by a prior study using payroll data (32). The cumulated number of quick returns during the first 32 pregnancy weeks was categorized as 0, 1–8 or ≥9 quick returns (roughly corresponded to ≥1 quick return per pregnancy month on average).
Reference group. In all analyses, we used two reference groups, fixed day work for comparisons of night versus day work, and the lowest category of exposure for within night work comparisons.
Outcome
The outcome severe PPD was identified by ICD-8 (296.0 – involutional melancholia – and 300.4 – depressive neurosis) or ICD-10 (F32 – depressive episode – and F33 – recurrent depressive disorder) codes registered as the primary diagnose at somatic or psychiatric hospital departments either as in- or outpatient treatment. As most of the women with perinatal psychiatric disorders in Denmark are treated in primary care (33), the outcome of our study was severe depression requiring in- and/or outpatient treatment at a hospital. The dates of registration were classified as prior (from 1969 to conception date) or postpartum (from birth to one year postpartum) in relation to each pregnancy.
Covariates
Covariates were chosen a priori based on data availability and previous literature (1, 8, 34, 35).
Age (<30, 30–35, >35 years), body mass index (BMI <25, 25.0–29.9, ≥30 kg/m2) and parity (1, 2, ≥3) registered by the midwife or family doctor at the first antenatal visit derived from the Danish Medical Birth Registry. The classification of socioeconomic status (SES) into high, low or medium derived from Statistics Denmark and was based on the two Danish versions of the International Standard Classification of Occupations from 2007–2009 and 2010-2015 (ISCO-88 and ISCO-08), respectively (36, 37). Sickness absence three months prior to pregnancy (0, 1–9 or ≥10 days) expressed the sum of all days registered with ≥3 hours of sickness absence in DWHD during this period. Prior diagnosed severe depression (yes/no) derived from the Danish National Patient Registry and the Danish Psychiatric Central Research Registry as outlined above.
Statistical analysis
We performed logistic regression of the risk of PPD by different dimensions of night work during the first 32 pregnancy weeks. We applied generalized estimating equations to account for repeated pregnancies within participants. We presented crude and adjusted estimates as odds ratio (OR) with 95% CI. Adjusted analyses included categories of age, BMI, SES, parity, sickness absence three months prior to pregnancy and prior diagnosed severe depression.
We performed the following sensitivity analyses: (i) restricted to nulliparous women; (ii) restricted to pregnancies without prior diagnosed severe depression; (iii) restricted to nurses, who represented most of the cohort; (iv) restricted to exposure in the first trimester; and (v) within-night work analysis with restriction to night work during the first or first and second trimester compared to night work throughout pregnancy. The reason for analyses (iv) and (v) was to evaluate the potential presence of healthy worker survivor effect in the main analysis. In another words, we accounted for the possibility of women, who are somehow susceptible to PPD, stopping night shift work during pregnancy and, therefore, not contributing to the group of workers with high cumulated exposure to night work.
We used a significance level of 0.05. The analyses were done with SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
The study cohort comprised 25 009 singleton pregnancies from 19 382 women. Their personal and working time characteristics are presented in Table 1. In around 61% of pregnancies, the woman worked ≥1 night shift during pregnancy. Among those, 64% were nurses and 17% were physicians, while most of the day workers were medical secretaries (18%), nurses (15%), and physiotherapists (14%). In only 84 (0.3%) pregnancies the working schedule was fixed night shifts. Night workers had on average 14.5 night shifts during the first 32 pregnancy weeks. Around 39% of night shifts lasted ≤8 hours, 32% lasted 9–12 hours and 29% >12 hours. Women who worked ≥1 spell of ≥4 consecutive night shifts had considerably higher number of consecutive night shifts in total (mean 17.7) compared to those with spells of 2–3 and no spells of ≥4 consecutive night shifts (mean 5.3). These categories represented therefore both length of spells and total number of consecutive night shifts. Even though quick returns after a night shift (shift intervals <28 hours) accounted for the compulsory day off following a night shift, they were more frequent than quick returns between any type of shifts (<11 hours) – see Table 1.
Table 1
Characteristics of 25 009 singleton pregnancies among 19 382 public hospital employees in Denmark, 2007–2015. [SD=standard deviation.]
| Day work a (N = 9642) | Night work b (N = 15 367) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| N | % | Mean | SD | N | % | Mean | SD | |
| Age (years) | 32.3 | 4.0 | 31.2 | 3.8 | ||||
| Body mass index (kg/m2) | 23.9 | 6.6 | 23.9 | 7.6 | ||||
| Parity | ||||||||
| 1 | 3128 | 32.9 | 6204 | 40.8 | ||||
| 2 | 4402 | 46.2 | 6169 | 40.5 | ||||
| ≥3 | 1990 | 20.9 | 2841 | 18.7 | ||||
| Socioeconomic status | ||||||||
| High | 2393 | 25.1 | 3541 | 23.1 | ||||
| Medium | 5390 | 56.6 | 10 818 | 70.7 | ||||
| Low | 1741 | 18.3 | 948 | 6.2 | ||||
| Most frequent occupations | ||||||||
| Laboratory technician | 709 | 7.4 | 395 | 2.6 | ||||
| Medical secretary | 1785 | 18.5 | 92 | 0.6 | ||||
| Nurse | 1447 | 15.0 | 9842 | 64.0 | ||||
| Nurse assistant | 279 | 2.9 | 801 | 5.2 | ||||
| Physician | 744 | 7.7 | 2654 | 17.3 | ||||
| Physiotherapist | 1358 | 14.1 | 44 | 0.3 | ||||
| Psychologist | 683 | 7.1 | 4 | 0.03 | ||||
| Type and number of shifts c | ||||||||
| Day | 107.4 | 30.0 | 60.6 | 26.7 | ||||
| Early morning | 0 | 0 | 0.04 | 0.9 | ||||
| Evening | 0 | 0 | 13.5 | 13.9 | ||||
| Night | 0 | 0 | 14.5 | 12.5 | ||||
| ≤8 hours | 0 | 0 | 5.7 | 10.3 | ||||
| 9–12 hours | 0 | 0 | 5.0 | 9.4 | ||||
| >12 hours | 0 | 0 | 3.8 | 7.0 | ||||
| Spells of 2–3 night shifts d | 0 | 0 | 5.3 | 4.5 | ||||
| Spells of ≥4 night shifts e | 0 | 0 | 17.7 | 11.4 | ||||
| Quick returns | 0 | 0 | 3.2 | 4.1 | ||||
| Quick returns after a night shift | 0 | 0 | 7.9 | 8.5 | ||||
| Weekly working hours e | 24.1 | 7.2 | 23.5 | 7.1 | ||||
| Sickness absence three months prior to pregnancy, days f | 2.9 | 7.0 | 2.6 | 5.4 | ||||
We identified 80 cases of PPD (0.3%) and 427 cases of prior diagnosed severe depression (1.7%). Approximately 20% of the women who developed PPD had a prior diagnose of severe depression, compared to 2% among women who did not develop PPD. Among women with prior diagnosed severe depression 4% developed PPD compared to 0.3% among those without a prior diagnosed severe depression.
Table 2 shows the results for the analysis of number of night shifts. Adjusted OR for PPD among women who worked ≥9 night shifts during the first 32 pregnancy weeks was 0.59 (95% CI 0.33–1.06) compared to day work and 0.66 (95% CI 0.36–1.23) compared to having 1-8 night shifts.
Table 2
Odds ratios (OR) of postpartum depression by number of night shifts during the first 32 pregnancy weeks among 19 382 public hospital employees in Denmark, 2007–2015. [CI=confidence interval]
| Number of night shifts | Pregnancies | Cases | Crude analysis | Adjusted analysis a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| N | % | N | % | OR | 95% CI | P for trend | OR | 95% CI | P for trend | |
| All pregnancies (N=25 009) | ||||||||||
| Day workb | 9642 | 38.5 | 36 | 0.4 | 1.00 | Referent | 0.06 | 1.00 | Referent | 0.08 |
| 1–8 | 5827 | 23.3 | 23 | 0.4 | 1.06 | 0.62–1.81 | 0.89 | 0.50–1.58 | ||
| ≥9 | 9540 | 38.2 | 21 | 0.2 | 0.59 | 0.34–1.02 | 0.59 | 0.33–1.06 | ||
| Pregnancies with ≥ 1 night shift during the first 32 pregnancy weeks (N=15 367) | ||||||||||
| 1–8 | 5827 | 37.9 | 23 | 0.4 | 1.00 | Referent | 1.00 | Referent | ||
| ≥9 | 9540 | 62.1 | 21 | 0.2 | 0.56 | 0.31–1.01 | 0.66 | 0.36–1.23 | ||
The results for the analysis of duration of night shifts are presented in Table 3. Adjusted OR for PPD among women who worked long night shifts (>12 hours) was 0.71 (95% CI 0.38–1.36) compared to day work and 0.76 (95% CI 0.29–1.97) compared to working only night shifts lasting ≤8 hours.
Table 3
Odds ratios (OR) of postpartum depression by duration of night shifts during the first 32 pregnancy weeks among 19 382 public hospital employees in Denmark, 2007–2015. [CI=confidence interval].
| Duration of night shifts | Pregnancies | Cases | Crude analysis | Adjusted analysis a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| N | % | N | % | OR | 95% CI | P for trend | OR | 95% CI | P for trend | |
| All pregnancies, N=19 125 | ||||||||||
| Day work b | 9642 | 50.4 | 36 | 0.4 | 1.00 | Referent | 0.31 | 1.00 | Referent | 0.26 |
| ≤8 hours c | 3427 | 17.9 | 10 | 0.3 | 0.78 | 0.38–1.59 | 0.74 | 0.35–1.55 | ||
| >12 hours d | 6056 | 31.7 | 17 | 0.3 | 0.75 | 0.42–1.35 | 0.71 | 0.38–1.36 | ||
| Pregnancies with ≥ 1 night shift during the first 32 pregnancy weeks, N=9483 | ||||||||||
| ≤8 hours | 3427 | 36.1 | 10 | 0.3 | 1.00 | Referent | 1.00 | Referent | ||
| >12 hours | 6056 | 63.9 | 17 | 0.3 | 0.96 | 0.44–2.10 | 0.76 | 0.29–1.97 | ||
Table 4 presents the results for number of consecutive night shifts. Adjusted OR for PPD among women who worked ≥4 consecutive night shifts weeks was 0.54 (95% CI 0.22–1.30) compared to day work and 0.69 (95% CI 0.23–2.08) compared to working only single night shifts.
Table 4
Odds ratios (OR) of postpartum depression by consecutive night shifts during the first 32 pregnancy weeks among 19 382 public hospital employees in Denmark, 2007–2015. [CI=confidence interval]
| Consecutive night shifts | Pregnancies | Cases | Crude analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| N | % | N | % | OR | 95% CI | P for trend | OR | 95% CI | P for trend | |
| All pregnancies (N=25 009) | ||||||||||
| Day work b | 9642 | 38.5 | 36 | 0.4 | 1.00 | Referent | 0.12 | 1.00 | Referent | 0.08 |
| 1c | 5172 | 20.7 | 18 | 0.4 | 0.93 | 0.52–1.66 | 0.93 | 0.49–1.77 | ||
| 2–3 d | 7409 | 29.7 | 20 | 0.3 | 0.72 | 0.41–1.26 | 0.63 | 0.35–1.16 | ||
| ≥4 e | 2786 | 11.1 | 6 | 0.2 | 0.58 | 0.24–1.38 | 0.54 | 0.22–1.30 | ||
| Pregnancies with ≥ 1 night shift during the first 32 pregnancy weeks (N=15 367) | ||||||||||
| 1 | 5172 | 33.7 | 18 | 0.4 | 1.00 | Referent | 0.26 | 1.00 | Referent | 0.50 |
| 2–3 | 7409 | 48.2 | 20 | 0.3 | 0.78 | 0.41–1.47 | 0.79 | 0.35–1.80 | ||
| ≥4 | 2786 | 18.1 | 6 | 0.2 | 0.62 | 0.25–1.56 | 0.69 | 0.23–2.08 | ||
Table 5 presents the results for quick returns. Adjusted OR for PPD among women who worked with ≥9 quick returns was 0.75 (95% CI 0.26–2.18) compared to day work, and 0.76 (95% CI 0.24–2.38) compared to night work without quick return. Adjusted OR for PPD among pregnancies with ≥9 quick returns after a night shift was 0.57 (95% CI 0.27–1.19) compared to day work, and 0.29 (95% CI 0.11–0.72) compared to nigh work without quick return after a night shift.
Table 5
Odds ratios (OR) of postpartum depression by number of quick returns a and quick returns after a night shift b during the first 32 pregnancy weeks among 19 382 public hospital employees in Denmark, 2007–2015. [CI=confidence interval]
| Pregnancies | Cases | Crude analysis | Adjusted analysis c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| N | % | N | % | OR | 95% CI | P for trend | OR | 95% CI | P for trend | |
| Quick returns, all pregnancies (N=25 009) | ||||||||||
| Day work d | 9642 | 38.5 | 36 | 0.4 | 1.00 | Referent | 0.09 | 1.00 | Referent | 0.07 |
| 0 e | 4605 | 18.4 | 19 | 0.4 | 1.11 | 0.63–1.95 | 1.05 | 0.57–1.93 | ||
| 1–8 | 9274 | 37.1 | 21 | 0.2 | 0.61 | 0.35–1.05 | 0.54 | 0.30–0.96 | ||
| ≥9 | 1488 | 6.0 | 4 | 0.3 | 0.72 | 0.25–2.03 | 0.75 | 0.26–2.18 | ||
| Pregnancies with ≥ 1 night shift during the first 32 pregnancy weeks (N=15 367) | ||||||||||
| 0 | 4605 | 30.0 | 19 | 0.4 | 1.00 | Referent | 0.16 | 1.00 | Referent | 0.30 |
| 1–8 | 9274 | 60.3 | 21 | 0.2 | 0.55 | 0.29–1.02 | 0.54 | 0.27–1.08 | ||
| ≥9 | 1488 | 9.7 | 4 | 0.3 | 0.65 | 0.22–1.91 | 0.76 | 0.24–2.38 | ||
| Quick returns after night shift, all pregnancies (N=25 009) | ||||||||||
| Day work | 9642 | 38.5 | 36 | 0.4 | 1.00 | Referent | 0.06 | 1.00 | Referent | 0.04 |
| 0 | 1078 | 4.3 | 8 | 0.7 | 2.00 | 0.92–4.33 | 1.99 | 0.89–4.48 | ||
| 1–8 | 9159 | 36.6 | 25 | 0.3 | 0.73 | 0.43–1.23 | 0.63 | 0.36–1.09 | ||
| ≥9 | 5130 | 20.5 | 11 | 0.2 | 0.57 | 0.29–1.14 | 0.57 | 0.27–1.19 | ||
| Pregnancies with ≥ 1 night shift during the first 32 pregnancy weeks (N=15 367) | ||||||||||
| 0 | 1078 | 7.0 | 8 | 0.7 | 1.00 | Referent | 0.06 | 1.00 | Referent | 0.10 |
| 1–8 | 9159 | 59.6 | 25 | 0.3 | 0.37 | 0.16–0.81 | 0.29 | 0.13–0.66 | ||
| ≥9 | 5130 | 33.4 | 11 | 0.2 | 0.29 | 0.12–0.72 | 0.29 | 0.11–0.72 | ||
Sensitivity analyses
Analysis restricted to nulliparous women (N=9332) did not substantially change the results from the main analyses (results not shown).
Analysis restricted to pregnancies without prior diagnosed severe depression (N=24 582) revealed adjusted OR 0.60 (95% CI 0.33–1.11) for women who worked ≥9 night shifts; OR 0.61 (95% CI 0.30–1.25) for women who worked long night shifts; OR 0.57 (95% CI 0.22–1.47) for women who worked ≥4 consecutive night shifts; OR 0.42 (95% CI 0.10–1.75) for women who worked ≥9 quick returns; and OR 0.59 (95% CI 0.28–1.28) for women who worked ≥9 quick returns after a night shift.
Analysis restricted to nurses (N=11 298) tended to an increased risk of PPD among women in the lowest categories of exposure compared with day work (adjusted OR 1.49, 95% CI 0.44–5.04 for 1–8 night shifts; OR 1.12, 95% CI 0.31–4.05 for night shifts of ≤8 hours; OR 1.27, 95% CI 0.31–5.10 for night work without consecutive night shifts; OR 1.56, 95% CI 0.42–5.82 for night work without quick returns; and OR 1.25, 95% CI 0.22–6.97 for night work without quick returns after a night shift).
Analysis restricted to exposure during the first trimester revealed overall slightly increased estimates, especially in comparisons of night versus day work – see supplementary tables (www.sjweh.fi/show_abstract.php?abstract_id=3831).
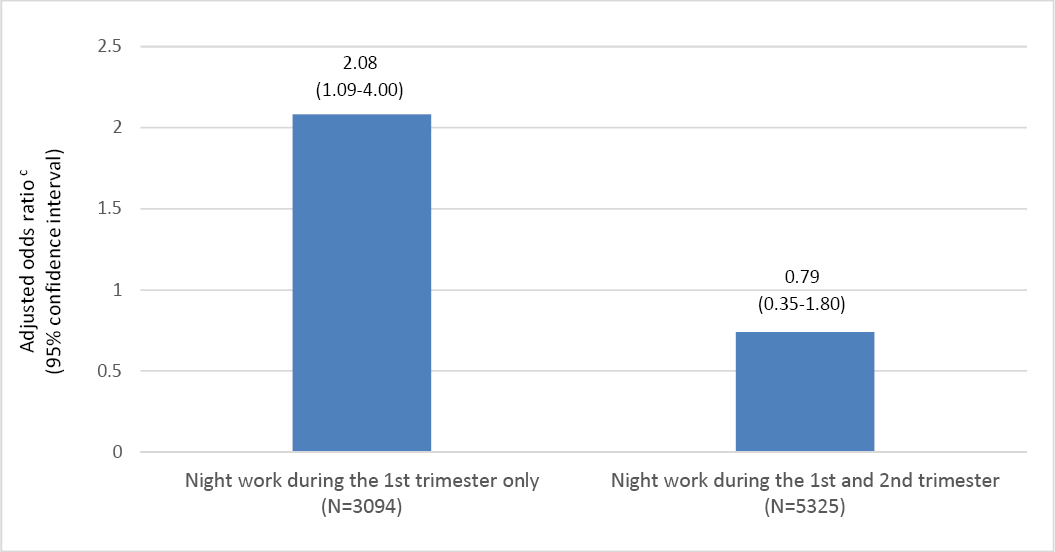
Within-night work sensitivity analysis revealed that women who stopped working night shifts after the first trimester (N=3094) presented with an increased risk of PPD (adjusted OR 2.08 95% CI 1.09–4.00) compared to women who worked night shifts throughout pregnancy – see figure 2. The risk of PPD was not increased (adjusted OR 0.79, 95% CI 0.35–1.80) among women who stopped working night shifts after the second trimester (N=5325). Women who stopped working night shifts after the first trimester had lower proportion of high SES (14% versus 25%) but had otherwise similar demographic characteristics as women with night work throughout pregnancy. Both groups had also similar prevalence of prior diagnosed severe depression (1.8 and 1.5% respectively).
Figure 2
Odds ratios of postpartum depression by night work aduring the first and second pregnancy trimester compared to night work throughout bpregnancy among 19 382 public hospital employees in Denmark, 2007–2015.
aAt least one night shift.
bAt least one night shift in each pregnancy trimester up to 32 pregnancy weeks.
cAdjusted for age, body mass index, socioeconomic status, parity, sickness absence three months prior to pregnancy and previously diagnosed severe depression.
Discussion
Overall, we did not find an increased risk of severe PPD in relation to night work during pregnancy in this nationwide cohort of Danish public hospital employees. We found, however, a 2-fold increased risk of PPD among women who stopped working night shifts after the first trimester.
To our knowledge, this is the first study investigating the association between different dimensions of night work during pregnancy and the risk of severe PPD. Our results are in line with prior longitudinal studies among hospital employees, which all found no overall association between night work and depression (38–45). Direct comparisons are not possible though as these studies applied self-reported information on depressive symptoms, not clinically diagnosed depression, and crude assessed exposure. The one study which accounted for changes of working schedules found that selection out of night work masked an effect of night work on the risk of common mental disorders (OR 1.25, 95% CI 1.03–1.52) (43). Similarly, the lack of association between PPD and the different dimensions of night work observed in our study can, at least partially, be explained by potential selection out of night work of susceptible individuals, ie, the healthy worker survivor effect. This is supported by findings of increased risk of severe PPD among women who stopped working night shifts after the first trimester (figure 2), and slightly increased estimates in analysis restricted to exposure during the first trimester (supplementary tables). The latter analysis excluded though a potential effect of night work during the second and trimester on the risk of PPD. The different categories of night work in our study were expressed by the cumulated exposure during the first 32 pregnancy weeks. Accordingly, the women with the highest exposure were those who endured night work throughout pregnancy. These women had, presumably, a relatively higher health status. As pointed out by Nabe-Nielsen and colleagues (46), their study surprisingly shows that shift workers had better mental health than day workers “choosing night work might be a deliberate choice based on personal needs and preferences” (46). It is however important to acknowledge that we did not have information on the reason for changing working schedules among women who stopped working night shifts after the first trimester. We can only hypothesize that these women were somehow at higher risk of developing PPD. It is possible that women in our cohort, as healthcare professionals, were more aware of the occurrence of depressive symptoms than other occupational groups. Nevertheless, a study of >12 000 workers from several sectors and trades in The Netherlands has shown that workers with depressive symptoms more often changed from shift to day work (relative risk 1.98, 95% CI 1.13–3.47) and from shift work to sick leave (relative risk 2.96, 95% CI 2.00–4.29) (45). Another study using the same cohort investigated changes in mental health as predictors of changes in working time and found that workers with prolonged fatigue, need for recovery or psychological distress tended to change from shift to day work (OR 3.44, 95% CI 1.42–8.38; OR 1.36, 0.34–5.45; and OR 2.26, 0.84–6.04, respectively) (47). Finally, a Finish study of >30 000 workers from different occupations (“from semi-skilled cleaners to physicians and mayors’”) found that night workers who developed common mental disorders had increased odds of moving to non-night work (OR 1.68, 95% CI 1.30–2.17) (43). One possible explanation for the association between depression and changes of working schedules is that worsening of depressive symptoms in relation to night work may be more easily recognizable than other disorders, such as hormonal dysfunction. Furthermore, adequate sleep is one of the non-pharmacological therapies in the treatment of depression (48).
It is important to identify women who work at night and who might be at risk of developing PPD, especially those who do not adapt to night work. As pregnant workers are not generally restrained from night work in Denmark, the identification of potential negative health effects of night work during pregnancy is based on individual clinical evaluation usually by a general practitioner, an obstetrician or an occupational physician. It has been shown that women with predisposition for depression may be more susceptible to hormonal and pro-inflammatory changes during pregnancy and postpartum period (17, 18, 20, 21). It is therefore possible that these women might also be more susceptible to hormonal changes and sleep disorders (22, 23, 25) induced by night work.
It is possible that the gradient of higher exposures corresponding to higher health status is also present within night work. In our study, this hypothesis is supported by findings of an increased risk of PPD among night workers without quick returns after a night shift (Table 5). Quick returns are frequently not part of planned schedules (49–51), but they might occur in periods of increased workload at the workplace. In this scenario, workers with poorer health status might not take extra shifts, avoiding quick returns. Also, analysis restricted to nurses, who represent a more homogeneous group regarding organization of night shifts, workload and job tasks, suggested elevated risks of PPD in the lowest categories of exposure to night work.
Further investigation of the healthy worker survivor effect among night workers requires specific epidemiological and statistical methods (14, 52–55). This would require information on the time of onset of depressive symptoms along with information on the reason for changing work schedules. Assessment of pregnancy period-specific exposure is also necessary as changes of working schedules occur generally during the end of the first trimester, when many women, at least in Denmark, inform their workplace about their pregnancy. Methods accounting for change of working schedules after the occurrence of disease, such as performed by Beltagy and colleagues (43), are suitable for accounting for changes of working schedule in relation to the occurrence of depressive symptoms during pregnancy. However, they are not applicable to PPD as it occurs per definition after birth, and therefore most probably when the woman is on maternity leave.
Strengths and limitations
The primary strengths of our study include nationwide sample size, prospective design, detailed and register-based exposure and outcome assessment, and within-night work comparisons.
Even though our cohort is nationwide, our results are based on relatively few cases as only the most severe cases of PPD are treated in hospitals in Denmark. As shown by Munk-Olsen and colleagues (33), for each birth resulting in inpatient psychiatric treatment of postpartum disorder in Denmark, 2.5 births resulted in outpatient treatment and 12 births resulted in general practitioner-provided pharmacological treatment. Therefore, even though the prevalence of severe PPD in our cohort (0.3%) corresponds to prior Danish population-based studies (33), low power is a limitation of our study. Misclassification of the mild cases of PPD might be an important source of bias towards the null in our cohort. The extent of this bias depends on whether night work is a risk factor specifically for mild cases of PPD. This is yet to be elucidated. Furthermore, it is possible that the low prevalence of severe PPD we observed among nurses and physicians might also reflect the possibility of receiving informal care from the work environment when having depressive symptoms. Nevertheless, including only severe cases of PPD increased the specificity of the outcome.
We lacked information on how well the participants adapted to night work. Psychological resilience (hardiness) and coping seem to be strongly related to psychological well-being (56). Further, we lacked information on chronotype. The evening chronotype, which is normally more frequent among night than day workers, has been independently associated with the risk and severity of depression (57). This might have biased the results towards the null if workers with evening chronotype, rather than other chronotypes, tended to change working schedules to non-night work due to depressive symptoms.
Concluding remarks
Overall, our results do not suggest that night work during pregnancy is a risk factor for severe PPD. However, we observed a 2-fold increased risk of PPD among women who stopped working night shifts after the first pregnancy trimester. This may reflect the influence of the healthy worker survivor effect, but the interpretation of this finding requires caution and warrants further attention.
Future research should include cases of PPD not requiring hospital treatment, use pregnancy period-specific exposure assessment, and apply epidemiological and statistical methods that account for the healthy worker survivor effect.