Asthma is one of the most frequent chronic diseases in children (1). Asthma symptoms mostly start in early childhood (2), indicating that the key period for asthma development occurs in utero or early childhood. The literature suggests that early-life exposures may influence the development of asthma, and prenatal stress has been flagged as one of these factors (3).
Prenatal stress may impact the hypothalamic-pituitary axis (4), and hence, may lead to dysregulation of immune system function (5–7). Most published studies suggested that prenatal exposure to life stressors was associated with increased risk of asthma (8–14), whereas other studies reported no association (15, 16). Moreover, inconsistencies exist regarding the strength of the association, probably due to differences in the type of stress indicators, asthma measurement (prevalence versus incidence), and the inclusion of children at different ages.
Asthma is not a single disease entity but encompasses several phenotypes, such as early-onset transient, early-onset persistent, and late-onset asthma (17). So far, most published studies addressed private life stressors (8–16), and the relative importance of work-related psychosocial factors during pregnancy on offspring asthma is not well understood. Only one study extended the focus to maternal self-reported job strain and the association to parent-reported asthma with an inherent risk of reporting bias (18). Furthermore, this study did not account for private life stressors.
Asthma is more frequent among boys in early childhood, but the gender gap reverses after puberty (19). This may be partly explained by sex-specific differences in vulnerability to environmental exposures, such as differential fetal-placental response to cortisol (20). Two studies suggested that boys are more vulnerable to stress during the prenatal period (8, 16), while a third study observed increased risk only in girls (9). To our knowledge, no study has considered co-exposure to both life and job stressors and how offspring sex might modify the associations in one single study, probably due to limited sample sizes and lack of appropriate measurement of job stressors.
To address these unknowns, we conducted a cohort study through linkages of Danish nationwide registers in combination with job exposure matrices (JEM) for job stressors from the Danish Work Environment Cohort Study (DWECS) (21). We aimed to examine the association between maternal exposure both to private life and job stressors prenatally and risk of offspring early-onset transient, early-onset persistent, and late-onset asthma, taking into account possible gender-specific interactions.
Methods
Study population
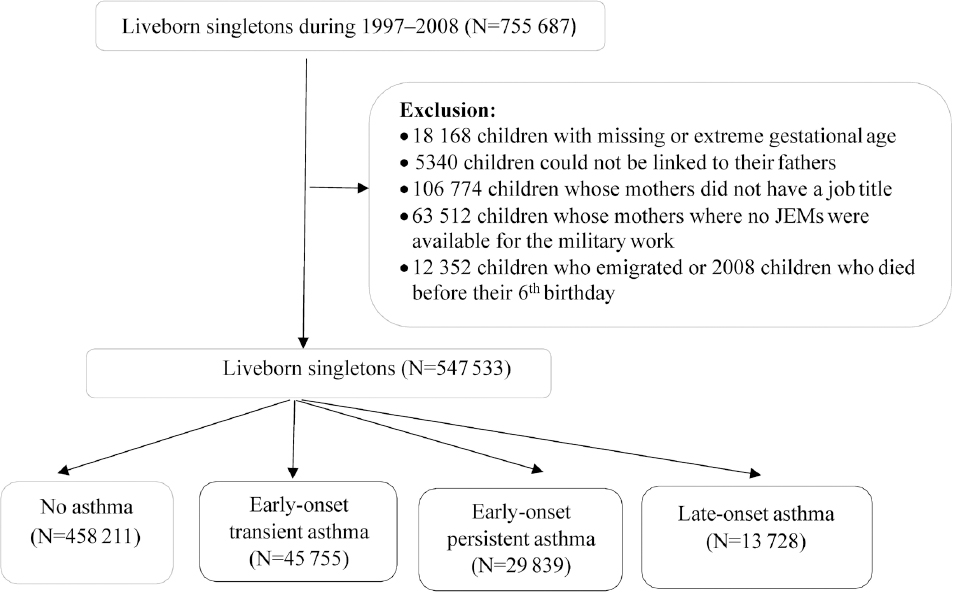
To conduct a population-based cohort study, we linked several Danish nationwide registers using the unique personal identification number, which is assigned to all liveborn and residents in Denmark. We identified 755 687 live singletons born between 1 January 1997 and 31 December 2008, recorded in the Danish Medical Birth Registry (DMBR) (22). We excluded 18 168 children with missing or extreme gestational age (<154 or >315 days), 5340 children with missing or erroneous linkage to their fathers, 106 774 children whose mothers did not have a job title at conception, and 63 512 children whose mothers worked in the military where no JEM were available. Since the children were followed until their 6th birthday, we further excluded 12 352 children who emigrated and 2008 children who died before their 6th birthday. The final analyses included 547 533 children (figure 1).
Life stressors and job stressors – exposure
Life stressors due to negative life events. We defined life stressors as negative life events occurring among the mothers from one year before conception until delivery. Negative life events included an array of events, such as death, life-threatening illness (cancer, ICD-10 codes C00–97, or acute myocardial infarction, ICD-10 codes I21–23), suicide attempt (ICD-10 codes X60–84, and Y87.0), or alcohol or drug abuse/dependence (ICD-10 codes F10–19) in a family member on the mother’s side (partner, child, parent, or sibling). The mother was linked to her family members via the Danish Civil Registration System (23). Information on life-threatening illness was retrieved from the Danish National Patient Register (24), death from the Danish Civil Registration System, and suicide attempt and alcohol or drug abuse/dependence from the Danish Psychiatric Central Research Register (25).
Psychosocial job demands and job control. Psychosocial JEM were used to define job stressors (26). We retrieved codes for the occupation held at the time of conception, coded according to the Danish version of the International Standard for Classification of Occupations (DISCO 88) from Statistics Denmark’s Integrated Database for Labor Market Research (27). This classification system is based on the required education/skills combined with the actual job task performed. The DISCO code consists of four hierarchical digits, where each additional digit indicates a more specific job category. The psychosocial JEM were created from DWECS data and provide information on sex-specific level of exposure for each DISCO code. Since 1990, the National Institute of Occupational Health conducted a telephone interview regarding psychosocial work environment among a representative random sample retrieved from the Danish central population register every fifth year, and the JEM used in the present study were created based on data collected in the year 2000 (21). We examined the components of job strain model (28), ie, job control and psychosocial job demands (hereinafter referred to as job demands) as well as the interactions between job control and job demands. Job demands consist of 3 items, and job control consists of decision authority (4 items) and skill discretion (4 items) (detailed items and response categories are provided in table S1, www.sjweh.fi/show_abstract.php?abstract_id=3785). Responses were scored with equal weight and intervals between answer probabilities and transformed to a 0–100 scale (29), with low scores indicating low levels of the measured dimension. Then the score for each DISCO occupational group was calculated based on the mean value of individual scores from this sample. We used scores only when the job category was represented by at least five individuals. Otherwise, a score with less specific job category (ie, fewer digit DISCO code) was used. We dichotomized job demands and control by the median value. We considered the exposure level as low if it fell below the median and high if it was above the median. For job control, we used the high exposure level as a reference, while the low exposure constituted the reference for job demands.
Childhood asthma phenotypes–outcome
Outcomes of interest were early-onset transient asthma, early-onset persistent asthma, and late-onset asthma based on hospital contact or pharmacological treatment for asthma. Asthma hospital contact was identified based on ICD-10 codes (J45 and J46) in the Danish National Patient Register. Anti-asthmatic treatment was retrieved from the Danish National Prescription Registry (30). The Anatomical Therapeutical Chemical classification codes for anti-asthmatic drugs were: inhaled β2-agonists (R03AC02, R03AC03, R03AC04, R03AC12 and R03AC13), inhaled glucocorticoids (R03BA01, R03BA02, and R03BA05), fixed-dose combination of inhaled β2-agonists and glucocorticoids (R03AK06 and R03AK07), leukotriene receptor antagonists (R03DC03), and anti-IgE treatment (R03DX05) (31).
We defined asthma treatment as ≥2 prescriptions of anti-asthmatic medications within a one-year period or ≥1 hospital contact for asthma (31). We categorized children into four mutually exclusive groups: (i) No asthma: no asthma treatment; (ii) early-onset transient asthma: asthma treatment during 0–3 years of age; (iii) early-onset persistent asthma: asthma treatment both at 0–3 years and 4–6 years of age; (iv) late-onset asthma: asthma treatment during 4–6 years of age.
Statistical analysis
We used log-binomial regression to estimate the prevalence ratios (PR) and 95% confidence intervals (CI) for each asthma phenotype by maternal exposure to negative life events, high job demands, and low job control prenatally. We then included an interaction term on the multiplicative scale for job demands × job control. Robust standard errors were used to account for some mothers contributing with more than one live singleton. The models were adjusted for a priori defined putative confounders: maternal age at delivery (<25, 25–34, or ≥35 years), education at conception (vocational education or below/higher education), smoking during pregnancy (yes/no), parity (1st/2nd or higher), comorbidity before delivery (yes/no), parental atopic status (yes/no), and calendar year of birth (1997–2000, 2001–2004, or 2005–2008). Mothers were considered to have a chronic disease if they had a disease listed in the Charlson Comorbidity Index (including 19 chronic disease categories excluding asthma) (32). We defined parental atopic disposition if any of the following diagnostic groups were recorded: allergic rhinitis without bronchial asthma (507 in ICD-8 and J30.1, J30.2, J30.3, or J30.4 in ICD-10), asthma (493 in ICD-8 and J45–J46 in ICD-10), or atopic dermatitis (691.0 in ICD-8 and L20 in ICD-10). Data on these covariates were extracted from the DMBR, the Danish National Patient Register, and the Danish Psychiatric Central Research Register. In the adjusted models, we mutually adjusted for negative life events, job demands, and job control. About 13.8% of the values were missing for any of the potential confounders, and we applied 20 imputations using the Markov Chain Monte Carlo technique for imputing missing values (33). To test for sex-specific associations, we repeated all analyses stratified by the sex of the child. Analyses were conducted in Stata 15 (Stata Corp, College Station, TX, USA).
We conducted five sensitivity analyses to estimate the robustness of the results. First, to account for confounding by maternal pre-pregnancy body mass index (BMI), we restricted our analyses to children born during 2003–2008 (N=209 097) when the information on pre-pregnancy BMI (≤18.5, 18.5–24.9, and ≥25 kg/m2) became available in the DMBR (22), and further adjusted for pre-pregnancy BMI. Second, the association between prenatal stress and asthma varies in the context of parental atopic predisposition. We, therefore, by stratification, examined whether the associations were affected by parental atopic status. Third, mothers with higher education often had higher job demands and control, according to our preliminary analysis. We, therefore, repeated our analyses by stratification on educational status. Fourth, to test whether the associations were affected by the cut-offs, we repeated the analyses by comparing the highest quintile versus below highest quintile of job demands and the lowest quintile versus above lowest quintile of job control. Fifth, early-onset transient asthma may vary with maternal smoking and age at delivery. To test whether these two covariates act as modificators instead of confounders in the associations between maternal negative life events, job stressors and offspring early-onset transient asthma, we further analyzed the data stratified by smoking status and maternal age.
Results
Table 1 presents the prevalence of asthma phenotypes according to the characteristics. During the follow-up, we documented 45 755 children with early-onset transient asthma, 29 839 with early-onset persistent asthma, and 13 728 with late-onset asthma. Several covariates were associated with increased risk of prevalence of childhood asthma: young age, low education, smoking during pregnancy, chronic disorder before delivery in mothers as well as parental atopic history.
Table 1
Prevalence of asthma phenotypes according to baseline descriptive statistics of the study population.
| Characteristic | No asthma (N=458 211) | Early-onset transient asthmaa(N=45 755) | Early-onset persistent asthmab (N=29 839) | Late-onset asthmac(N=13 728) |
|---|---|---|---|---|
|
|
|
|
|
|
| N (%) | N (%) | N (%) | N (%) | |
| Maternal age at delivery (years) | ||||
| <25 | 44 279 (80.3) | 6015 (10.9) | 3234 (5.9) | 1601 (2.9) |
| 25–34 | 334 552 (83.6) | 33 538 (8.4) | 22 072 (5.5) | 9959 (2.5) |
| ≥35 | 79 380 (86.0) | 6202 (6.7) | 4533 (4.9) | 2168 (2.3) |
| Maternal education at conception | ||||
| Vocational education or below | 268 715 (82.6) | 29 794 (9.2) | 18 346 (5.6) | 8370 (2.6) |
| Higher education | 185 766 (85.2) | 15 685 (7.2) | 11 297 (5.2) | 5253 (2.4) |
| Missing | 3730 (86.6) | 276 (6.4) | 196 (4.6) | 105 (2.4) |
| Maternal smoking during pregnancy | ||||
| No | 334 122 (84.3) | 30 975 (7.8) | 21 426 (5.4) | 9826 (2.5) |
| Yes | 65 035 (78.9) | 10 040 (12.2) | 5305 (6.4) | 2093 (2.5) |
| Missing | 59 054 (85.9) | 4740 (6.9) | 3108 (4.5) | 1809 (2.6) |
| Maternal comorbidity before delivery | ||||
| No | 443 215 (83.8) | 43 871 (8.3) | 28 515 (5.4) | 13 191 (2.5) |
| Yes | 14 996 (80.0) | 1884 (10.1) | 1324 (7.1) | 537 (2.9) |
| Parity | ||||
| 1st | 204 604 (83.6) | 19 296 (7.9) | 13 998 (5.7) | 6868 (2.8) |
| ≥2 | 251 004 (83.8) | 26 187 (8.7) | 15 631 (5.2) | 6786 (2.3) |
| Missing | 2603 (82.4) | 272 (8.6) | 210 (6.6) | 74 (2.3) |
| Parental atopic status | ||||
| No | 431 164 (84.3) | 41 725 (8.2) | 26 161 (5.1) | 12 228 (2.4) |
| Yes | 27 047 (74.6) | 4030 (11.1) | 3678 (10.1) | 1500 (4.1) |
| Sex of the child | ||||
| Boy | 227 092 (80.8) | 27 798 (9.9) | 18 366 (6.5) | 7965 (2.8) |
| Girl | 231 119 (86.8) | 17 957 (6.7) | 11 473 (4.3) | 5763 (2.2) |
| Calendar year of birth | ||||
| 1997–2000 | 162 519 (85.9) | 13 049 (6.9) | 8682 (4.6) | 5011 (2.6) |
| 2001–2004 | 150 837 (83.6) | 14 699 (8.1) | 10 251 (5.7) | 4689 (2.6) |
| 2005–2008 | 144 855 (81.5) | 18 007 (10.1) | 10 906 (6.1) | 4028 (2.3) |
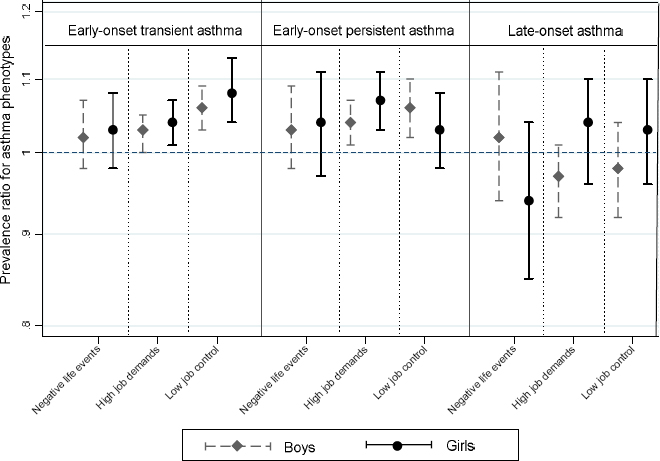
In the adjusted models, maternal prenatal exposure to negative life events was not statistically significantly associated with increased risk of early-onset transient asthma (PR=1.02, 95% CI 0.99–1.06), early-onset persistent asthma (PR=1.04, 95% CI 0.99–1.08), or late-onset asthma (PR=0.99, 95% CI 0.93–1.05) in offspring. Maternal exposure to high job demands or low job control was associated with increased risk of early-onset transient and persistent asthma in offspring (table 2). The associations mentioned above were not altered by sex of the child (figure 2), or parental atopic history (supplementary figure S1, www.sjweh.fi/show_abstract.php?abstract_id=3785).
Table 2
Prevalence ratio (PR) for asthma phenotypes according to prenatal exposure to job stressors and negative life events (main effects). [CI=confidence interval.]
| Characteristic | N | Early-onset transient asthma | Early-onset persistent asthma | Late-onset asthma | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Cases N (%) | Crude PR | Adjusted PRa (95% CI) | Cases N (%) | Crude PR | Adjusted PRa(95% CI) | Cases N (%) | Crude PR | Adjusted PRa(95% CI) | ||
| Negative life events | ||||||||||
| No | 506 101 | 42 217 (8.3) | 1 | 1 (ref) | 27 520 (5.4) | 1 | 1 (ref) | 12 713 (2.5) | 1 | 1 (ref) |
| Yes | 41 432 | 3538 (8.5) | 1.02 | 1.02 (0.99–1.06) | 2319 (5.6) | 1.03 | 1.04 (0.99–1.08) | 1015 (2.5) | 0.98 | 0.99 (0.93–1.05) |
| Job demands | ||||||||||
| Low | 292 080 | 25 151 (8.6) | 1 | 1 (ref) | 15 815 (5.4) | 1 | 1 (ref) | 7375 (2.5) | 1 | 1 (ref) |
| High | 255 453 | 20 604 (8.1) | 0.94 | 1.03 (1.01–1.05) | 14 024 (5.5) | 1.01 | 1.05 (1.02–1.07) | 6353 (2.5) | 0.98 | 1.00 (0.96–1.03) |
| Job control | ||||||||||
| Low | 292 068 | 26 854 (9.2) | 1.24 | 1.07 (1.04–1.09) | 16 514 (5.7) | 1.08 | 1.05 (1.02–1.08) | 7482 (2.6) | 1.05 | 1.00 (0.95–1.04) |
| High | 255 465 | 18 901 (7.4) | 1 | 1 (ref) | 13 325 (5.2) | 1 | 1 (ref) | 6246 (2.4) | 1 | 1 (ref) |
Figure 2
Prevalence ratio for asthma phenotypes according to prenatal exposure to job stressors and negative life events stratified by gender of the child. Adjusted for material age at delivery, maternal highest education at conception, smoking during pregnancy, chronic disorders, parity, parental atopic disorders, and calendar year of birth. Negative life events, job control and job demands were mutually adjusted for in the models.
In the fully adjusted models including the interaction term on the multiplicative scale for job demands × job control, there was evidence of interaction for all three asthma phenotypes (all P-values <0.001 for the interaction). Among children of mothers with low job demands, low job control was associated with higher risk of all three asthma phenotypes; the PR was 1.14 (95% CI 1.09–1.19) for early-onset transient asthma, 1.17 (95% CI 1.11–1.23) for early-onset persistent asthma, and 1.06 (95% CI 1.00–1.14) for late-onset asthma, compared to high job control. Among children of mothers with high job demands, low job control was not significantly associated with offspring asthma phenotypes except for early-onset persistent asthma where a reduced risk was observed (PR=0.94, 95% CI 0.90–0.97), in comparison to high job control (table 3). The associations above were not modified by child sex (table 4), parental atopic history (table S2, supplementary material, www.sjweh.fi/show_abstract.php?abstract_id=3785), or maternal education status (table S3, supplementary material, www.sjweh.fi/show_abstract.php?abstract_id=3785).
Table 3
Prevalence ratio (PR) for asthma phenotypes in the offspring according to maternal job demands and job control. [CI=confidence interval.]
| Job control | Low job demands | High job demands | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | Cases N (%) | Adjusted PR (95% CI)a | N | Cases N (%) | Adjusted PR (95% CI)a | |
| Early-onset transient asthma | ||||||
| Low job control | 190 481 | 18 432 (9.7) | 1.14 (1.09–1.19) | 101 587 | 8422 (8.3) | 1.00 (0.97–1.03) |
| High job control | 101 599 | 6719 (6.6) | 1 (ref) | 153 866 | 12 182 (7.9) | 1 (ref) |
| Early-onset persistent asthma | ||||||
| Low job control | 190 481 | 11 240 (5.9) | 1.17 (1.11–1.23) | 101 587 | 5274 (5.2) | 0.94 (0.90–0.97) |
| High job control | 101 599 | 4575 (4.5) | 1 (ref) | 153 866 | 8750 (5.7) | 1 (ref) |
| Late-onset asthma | ||||||
| Low job control | 190 481 | 5032 (2.6) | 1.06 (1.00–1.14) | 101 587 | 2450 (2.4) | 0.94 (0.88–1.01) |
| High job control | 101 599 | 2343 (2.3) | 1 (ref) | 153 866 | 3903 (2.5) | 1 (ref) |
Table 4
Adjusted prevalence ratio (PR) for asthma phenotypes in the offspring according to job demands and job control among children stratified by sex of the child. [CI=confidence interval.]
| Job control | Boys | Girls | ||
|---|---|---|---|---|
|
|
|
|||
| Low job demands | High job demands | Low job demands | High job demands | |
|
|
|
|
|
|
| Adjusted PRa (95% CI)a | Adjusted PRa (95% CI)a | Adjusted PRa (95% CI)a | Adjusted PRa (95% CI)a | |
| Early-onset transient asthma | ||||
| Low job control | 1.12 (1.07–1.18) | 0.99 (0.95–1.03) | 1.16 (1.09–1.24) | 1.01 (0.96–1.06) |
| High job control | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Early-onset persistent asthma | ||||
| Low job control | 1.17 (1.10–1.24) | 0.95 (0.91–1.00) | 1.17 (1.08–1.27) | 0.91 (0.85–0.97) |
| High job control | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Late-onset asthma | ||||
| Low job control | 1.01 (0.92–1.12) | 0.94 (0.87–1.02) | 1.13 (1.01–1.27) | 0.93 (0.85–1.02) |
| High job control | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
The associations remained largely unchanged when we adjusted for pre-pregnancy BMI (data not shown). When we use the quintile as the cutoffs of job demands and job control, the same direction of associations between job demands, job control, and offspring asthma phenotypes were observed, although the strength of the associations changed slightly (supplementary table S4). The associations between negative life events, job stressors and early-onset transient asthma were similar among children born by mothers at different age groups (all P-values >0.10 for the interactions of exposures × maternal age at delivery) (supplementary figure S2, www.sjweh.fi/show_abstract.php?abstract_id=3785). There was no strong evidence that maternal smoking during pregnancy modified the associations between negative life events, job control and offspring early-onset transient asthma (P>0.70 for the interactions of negative life events × smoking and job control × smoking), although maternal high job demands were associated with increased risk of early-onset transient asthma only among children born by nonsmoking mothers but not smoking mothers (P<0.01 for the interaction of job demands × smoking) (supplementary figure S3, www.sjweh.fi/show_abstract.php?abstract_id=3785).
Discussion
In this large nationwide study, there is no evidence of increased risk of asthma among children whose mothers were exposed to negative life events prenatally. Maternal low job control was associated with increased risk of all three asthma phenotypes among mothers with low job demands. Among women with high job demands, low job control was not associated with offspring asthma phenotypes apart from a reduced risk of early-onset persistent asthma. These associations were independent of child sex or parental atopic status.
Results from published studies on the association between prenatal exposure to negative life events and childhood asthma are inconsistent. Whereas some studies found full support for increased risk of asthma after maternal exposure to negative life events (8–14), another two population-based cohort studies found no consistent support (15, 16). The distinct type of stress indicators, timing of asthma diagnosis and asthma phenotypes could potentially explain seemingly conflicting findings from previous studies (3, 14). Our study does not support maternal exposure to negative life events influences offspring asthma. These findings are in line with our previous study examining different asthma phenotypes (15). However, the magnitude of risk estimates in our study was smaller than that reported by a meta-analysis by Flanigan et al (3), in which a relative risk of 1.15 (95% CI 1.04–1.27) for offspring asthma was reported for children exposed to stress prenatally. We defined life stressors according to an array of negative life events, such as death, life-threatening illness, alcohol or drug abuse/dependence of family members, which may reflect less severe stressors than the death of a child reported in our previous study (15).
We found the associations between job control and offspring asthma phenotypes were modified by job demands. The modifying effect of job demands on the associations may be explained by the difference in occupational categories, for instance, jobs with low demands are often manual work with physical objects and high demands with clients (34). Specifically, we found that low job control increased the risk of offspring asthma only among mothers with low job demands, whereas a reduced risk of early-onset persistent asthma was observed among mothers with high job demands. This finding did not support the hypothesized association with job control but is in line with an earlier Danish study (18), where Larsen et al found a higher risk of asthma among children whose mothers were exposed to self-reported high job demands and control but not high job demands and low job control. We have no adequate explanation of these associations and further mechanistic studies are warranted. We can only speculate the possible mechanism behind our findings. Mothers with low job control and high job demands are suggested to have elevated levels of cortisol than mothers with high job control and low job demands (35). Maternal cortisol can be transmitted to the fetus (36), resulting in Th2-biased cell differentiation in the fetus and increased susceptibility to asthma later in life (37). The other likely explanation apart from chance finding is confounding by socioeconomic status which is strongly related to job control, despite socioeconomic status is included in our analyses. Residential exposure to air pollution during pregnancy may be related to both offspring asthma phenotypes and job stressors since socioeconomically disadvantaged populations probably are more likely to be exposed to residential air pollution. It is possible that mothers with low job demands and low job control live in areas with more residential air pollution than children to mothers with low job demands and high job control, which would lead to an overestimation of the association between low job control and offspring asthma phenotypes as observed in our study. In a sensitivity analysis, we stratified by educational status and did see similar associations across the educational level, suggesting that our results cannot be explained by residual confounding alone.
Results from studies reporting sex-specific effect of prenatal stress on asthma are heterogeneous (8, 9, ,16). Our study did not disclose any gender differences in susceptibility to asthma relative to maternal exposures. This ambiguity may be explained by both offspring gender disparities in biology and the response to the stressors. For instance, surfactant production begins later in male than female neonatal lungs, contributing to lower airflow rate and higher airway resistance in boys (38). A recent study suggested that boys are more vulnerable to prenatal stress, whereas stress in the postnatal period was more significantly associated with asthma in girls (8). It is likely that prenatal stressors included in our study may be long-lasting and continue to influence mothers and their children in the postnatal period and thus dilute differential effects caused by child sex.
Methodological considerations
Our study is a population-based cohort study and includes the entire Danish population during the study period. Information on the exposure and outcome were collected independently and was therefore not affected by recall bias. The use of registers allowed us to control for some potential confounders. The use of JEM to assess job stress enables us to assess job demands and job control independently of individual self-reports and thus minimizes the risk of reporting bias, but at the expense of accuracy in the exposure assignment.
There are also some limitations. First, we only included children of mothers with DISCO codes and hence exclude 22.1% of children whose mothers did not have a job title at conception or worked in the military from the study population. Second, the perception and reaction, and coping strategies of a working environment may vary between individuals. We assigned a mean value of exposure to mothers with the same job titles while other factors such as coping skills may influence job stress. Furthermore, we used the job title at conception as one single measure of job stress throughout pregnancy. However, exposure to job stress may change during pregnancy since mothers may take sick leave or even leave the job when work-related stress is too intensive or causes severe health problems. Therefore, we may introduce non-differential misclassification and bias the effect estimates toward the null. Third, job control is correlated with other occupation exposures, which may have joint effects on offspring asthma phenotypes. We do not have direct information on other occupation exposures and thus were not able to include them in the models. Failure to adjust for the concomitant exposure would have biased our findings away from the null. However, similar results were obtained when we stratified by maternal education status which partly reflects the job titles and thus other occupational exposures. Therefore, the associations observed cannot entirely be ascribed to residual confounding by concomitant exposure. In future studies, it can be considered to include other occupational exposures via job exposure matrices, for instance, noise, particles, and chemical exposures. Fourth, although we accounted for some covariates in our models, residual confounding cannot be ruled out. Fifth, we did not have information on the atopic status of asthmatic children, and we were therefore not able to differentiate the association of maternal prenatal exposure to life and job stressors with allergic and non-allergic asthma in offspring.
Concluding remarks
Maternal stressors in private life do not influence offspring asthma significantly, whereas low job control is associated with offspring asthma phenotypes, which is modified by maternal psychosocial job demands.
Funding
The Danish Working Environment Research Foundation supported this study (17-2015-09 20150067134); Liu X is also supported by the Danish Council for Independent Research (Project No. DFF-5053-00156B). The funders had no roles in study design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors declare no conflicts of interest.